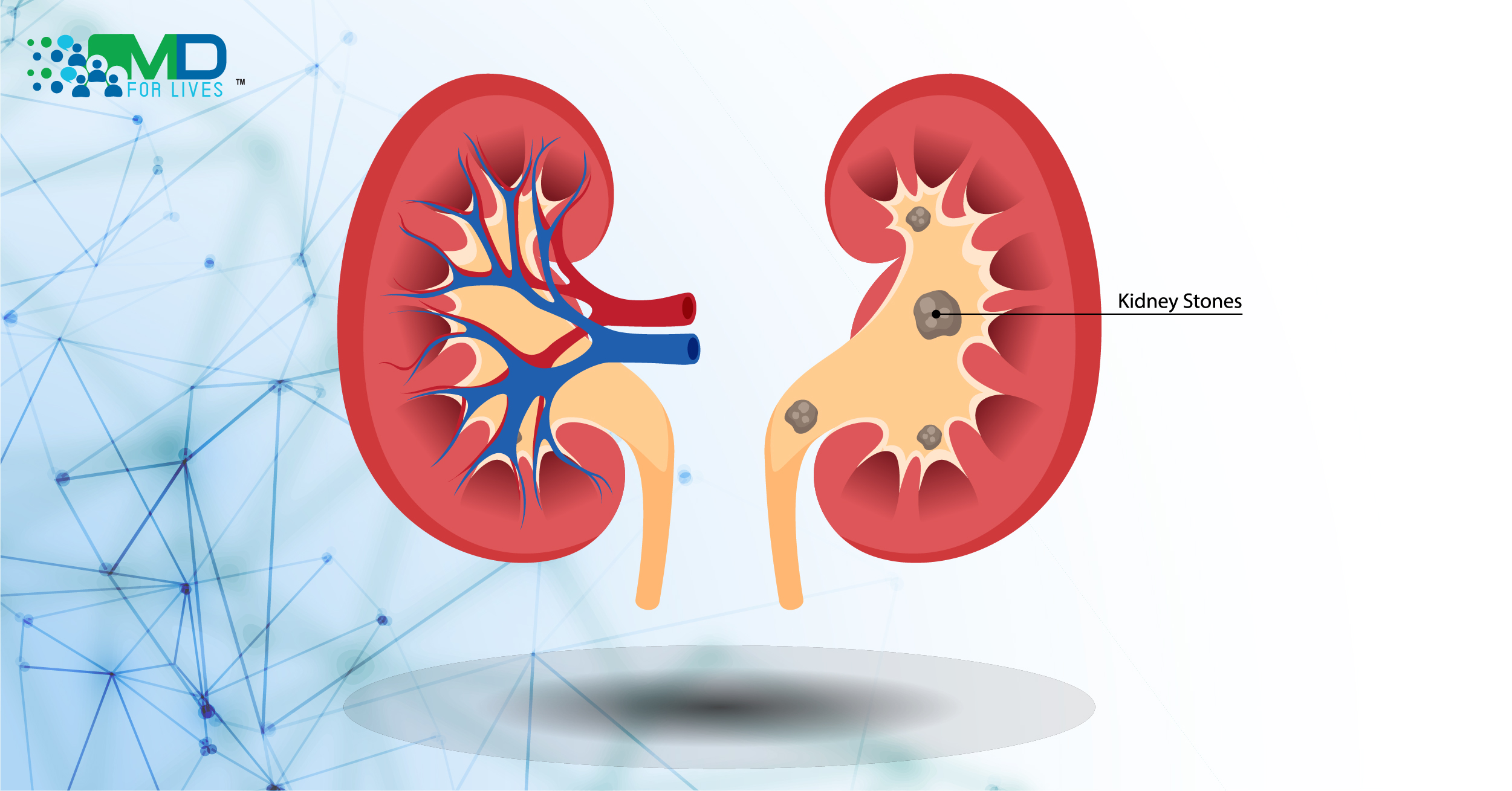
Primary hyperoxaluria (PH) is a rare genetic disease in which high levels of oxalate are produced and can deposit in the kidneys and other organs of the body.1 The disorder can eventually lead to kidney failure and, in severe cases, death.2
In late 2020, an RNAi agent called lumasiran was approved for the treatment of primary hyperoxaluria type 1 (PH1), a severe version of the condition.1 Last month, investigators reported that lumasiran successfully reduced urinary oxalate excretion to near-normal levels after 6 months in a group of PH1 patients.2
Cause and symptoms of hyperoxaluria
In PH, one of several genes encoding glyoxylate-metabolizing enzymes is mutated, leading to excess oxalate formation and consequent excretion in the urine.4 High levels of oxalate cause the formation of calcium oxalate stones in the kidneys.3 Depending on the severity of the disease, oxalate buildup can cause recurring kidney stones, kidney failure, and eventually oxalate accumulation in the bones, eyes, heart, skin, central nervous system, and blood vessels.3 Primary hyperoxaluria type 1, especially, is a life-threatening disease, and the resulting kidney failure can be fatal early in life.2

Genetic mutations in primary hyperoxaluria
The three types of primary hyperoxaluria, PH1, PH2, and PH3, each involves a different mutated gene.3,4 The gene involved in PH1, AGXT, encodes the hepatic peroxisomal enzyme alanine-glyoxylate aminotransferase (AGT).3 PH1 accounts for about 80% of all PH cases, and it is the most severe form.1,4
The GRHPR gene, which encodes the enzyme glyoxylate reductase/hydroxypyruvate reductase, and the HOGA1 gene, which encodes the enzyme 4-hydroxy-2-oxoglutarate aldolase, are involved in PH2 and PH3, respectively.3 All three forms of PH involve elevated levels of oxalate in the body.3,4
Standard primary hyperoxaluria treatment
Reducing the formation of stones in the kidneys is an important strategy to preserve kidney function.3 Drinking large amounts of water, known as hyperhydration, can reduce the formation of oxalate stones but is hard to maintain.2,3 In some cases, a gastrostomy may be needed to achieve adequate hydration, especially in children.2,3
Calcium oxalate crystallization inhibitors, in combination with hyperhydration, can reduce the formation of kidney stones.2,3 Pyridoxine (vitamin B6) is a coenzyme for AGT and as a supplement, it can help some patients with PH1.2,3 Once the disease progresses, intensive kidney dialysis, kidney or liver transplant, or double liver-kidney transplants may be required to save the patient.2,3 While transplants can improve patients’ outcomes, the procedures are risky and patients have to use immunosuppressive drugs for the rest of their lives.2

New primary hyperoxaluria treatment
Lumasiran (Oxlumo) became the first drug approved by the FDA to treat PH1 in November 2020.1 Lumasiran is an RNA interference (RNAi) therapy that inhibits the production of the hepatic enzyme glycolate oxidase (GO).2,3
Normally, AGT converts glyoxylate to glycine. When AGT is deficient, glyoxylate is either oxidized by GO to oxalate or reduced to glycolate.5 Lumasiran inhibits the production of GO enzyme, leading to less oxalate and more glycolate formation in the liver.2

The results from a double-blind phase 3 trial (ILLUMINATE-A) that was published in the New England Journal of Medicine in April 2021 showed that urinary oxalate levels in patients aged 6 to 60 years reached normal or near-normal levels after 6 months of treatment with lumasiran .2
The effect of lumasiran was seen within the first month of treatment, and the primary outcome, which is the percent change in urinary oxalate levels from baseline to month 6, was achieved after 6 months.2 A 65.4% reduction in urinary oxalate was seen in the lumasiran group compared to 11.8% in the placebo group.2 The plasma oxalate levels were also reduced in the lumasiran group, with a 39.5 percentage point difference between the two groups in plasma oxalate levels after 6 months.2 Another study, ILLUMINATE-B, was conducted in patients younger than 6 years old, and the efficacy and safety results at six months were similar to those from the ILLUMINATE-A study.6

Another investigational RNAi agent called nedosiran is in clinical trials as a possible treatment for all three types of PH.3,7 Nedosiran inhibits the formation of the hepatic enzyme lactate dehydrogenase type a (LDHa), which converts glyoxylate into oxalate, thus reducing liver production of oxalate.3,7 Interim results in patients with PH1 and PH2 showed normal or near-normal urinary oxalate levels after nedosiran injections.8
Other medications under investigation for primary hyperoxaluria treatment include stiripentol, which is an anti-epileptic medication that can block LDH-5, and Oxabact OC5, which is a biotherapeutic drug derived from oxalate-metabolizing bacteria.9,10,11