On April 22, 2021, the US Food and Drug Administration (FDA) granted Accelerated Approval to dostarlimab (Jemperli, GlaxoSmithKline) as an endometrial cancer treatment. Dostarlimab is approved specifically to treat recurrent or advanced mismatch repair-deficient (dMMR) endometrial cancer in patients who previously received platinum-based doublet chemotherapy.1
Dostarlimab is a monoclonal antibody that inhibits programmed cell death protein 1 (PD-1) and falls under the category of checkpoint inhibitors. PD-1 is a receptor found on T cells that downregulates immune responses when it binds to its ligands PD-L1 and PD-L2. PD-L1 is expressed at higher levels on some cancer cells, and this is thought to weaken the immune response against the tumor.2 Dostarlimab inhibits the binding of PD-1 to both of its ligands.3

Dostarlimab received Priority Review and Breakthrough Therapy designations for endometrial cancer treatment, attesting to its importance in filling an unmet need in a subset of patients with progressive disease. The efficacy of dostarlimab was evaluated based on the GARNET Clinical Trial (NCT02715284).4 The Accelerated Approval means the FDA may require the manufacturer to present further data once an ongoing Phase 3 clinical trial is completed.
Endometrial cancer epidemiology
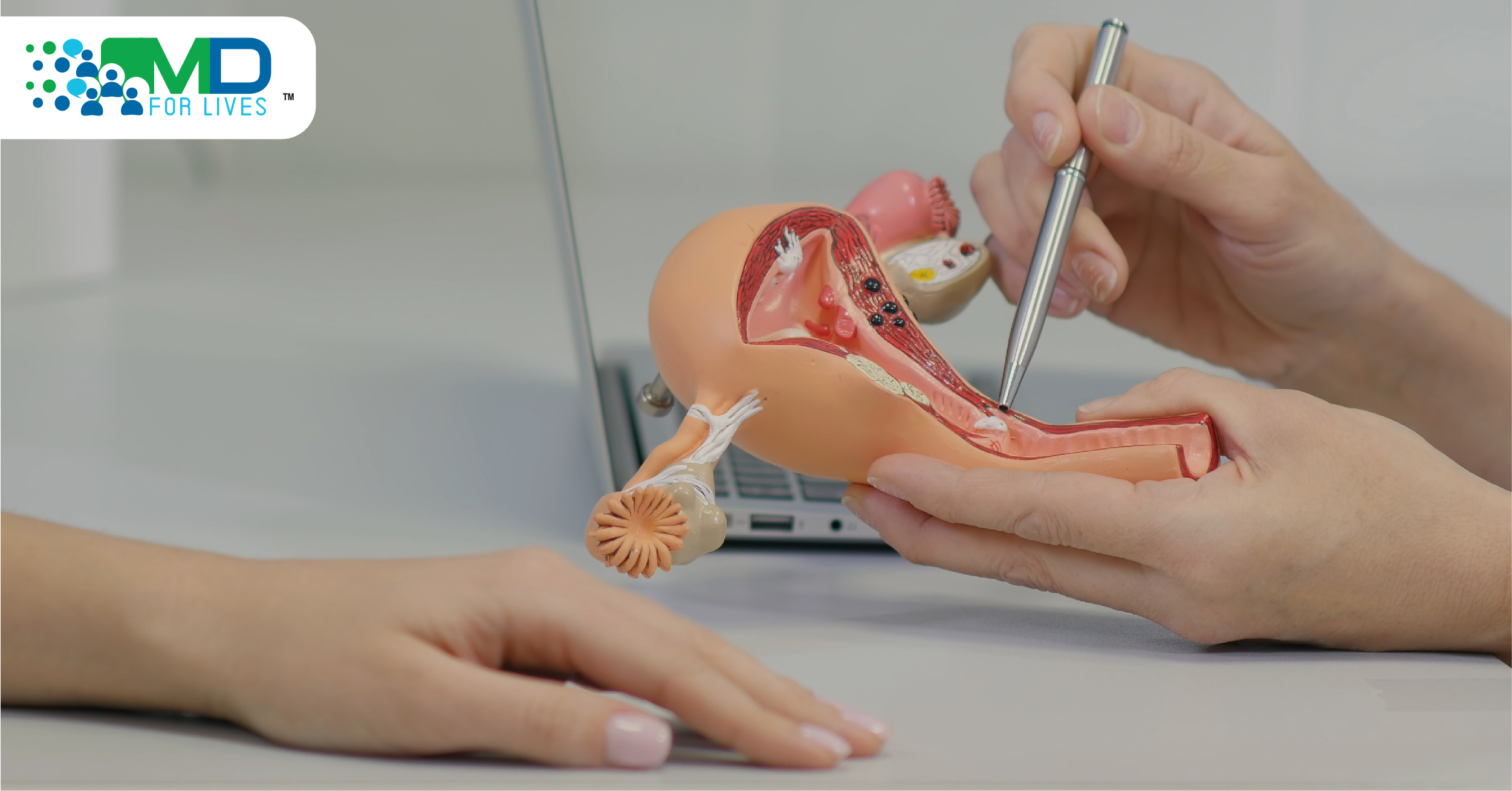
Endometrial cancer is cancer of the endometrium, the lining of the uterus. It is the most common form of uterine cancer and the most common gynecologic cancer in the US.5,6 Most cases of uterine cancer are diagnosed in women over age 55, and the median age at diagnosis is 63 years.5 Besides age, risk factors for developing endometrial cancer include hormone therapy, obesity, metabolic syndrome, diabetes, and genetic factors.7,8
According to data from the National Cancer Institute, Surveillance, Epidemiology, and End Results Program (SEER, 2018), it was estimated 813,861 women were living with uterine cancer in the US.5 Current SEER estimates for 2021 predict 66,570 new cases of uterine cancer and 19,940 deaths from this gynecologic cancer that affects women of all races.5
GARNET Phase 1 trial review
Results from the GARNET Phase 1 clinical study were published online in JAMA Oncology on October 1, 2020.3 This is an ongoing, non-randomized, open-label, multicenter Phase 1/2 study of dostarlimab in patients with recurrent or advanced endometrial cancer whose disease progressed after platinum-based doublet chemotherapy.
Of the 104 women enrolled (median age 64 years) with dMMR who received dostarlimab, 71 patients were included in the interim analysis. Patients received dostarlimab, 500 mg by intravenous infusion once every 3 weeks for 4 doses, then 1000 mg once every 6 weeks.3
The efficacy analysis demonstrated that 30 of the 71 patients had a confirmed response, for an overall response rate of 42.3% (95% CI, 30.6%-54.6%). The complete response rate (disappearance of the tumor) was 12.7% (n=9) and the confirmed partial response rate (shrinkage of the tumor) was 29.6% (n=21).3 The response had a duration of 6 months or longer in 93.3% of these patients.9
The most common grade 3 treatment-related adverse events (TRAEs) were anemia (3%), colitis (2%), and diarrhea (2%). Fewer than 2% of patients discontinued dostarlimab because of TRAEs.
Dostarlimab and current endometrial cancer treatments
Endometrial cancer diagnosis, classification, and staging require the histologic evaluation of the patient’s endometrial tissue, usually obtained by biopsy, curettage sample, or from a specimen procured during hysterectomy.10 Because 25% to 30% of patients with advanced endometrial cancer have dMMR tumors,1 biomarker testing for dMMR is recommended following diagnosis of uterine cancer.11 Other genetic testing and genetic counseling may be recommended.

The patient’s endometrial cancer treatment plan is based on risk stratification and may include surgery, radiation, and systemic therapies such as chemotherapy, hormone therapy, targeted therapy, and immunotherapy.11 If the endometrial cancer cells have dMMR and platinum-containing chemotherapy has already been tried, then dostarlimab immunotherapy may be considered.