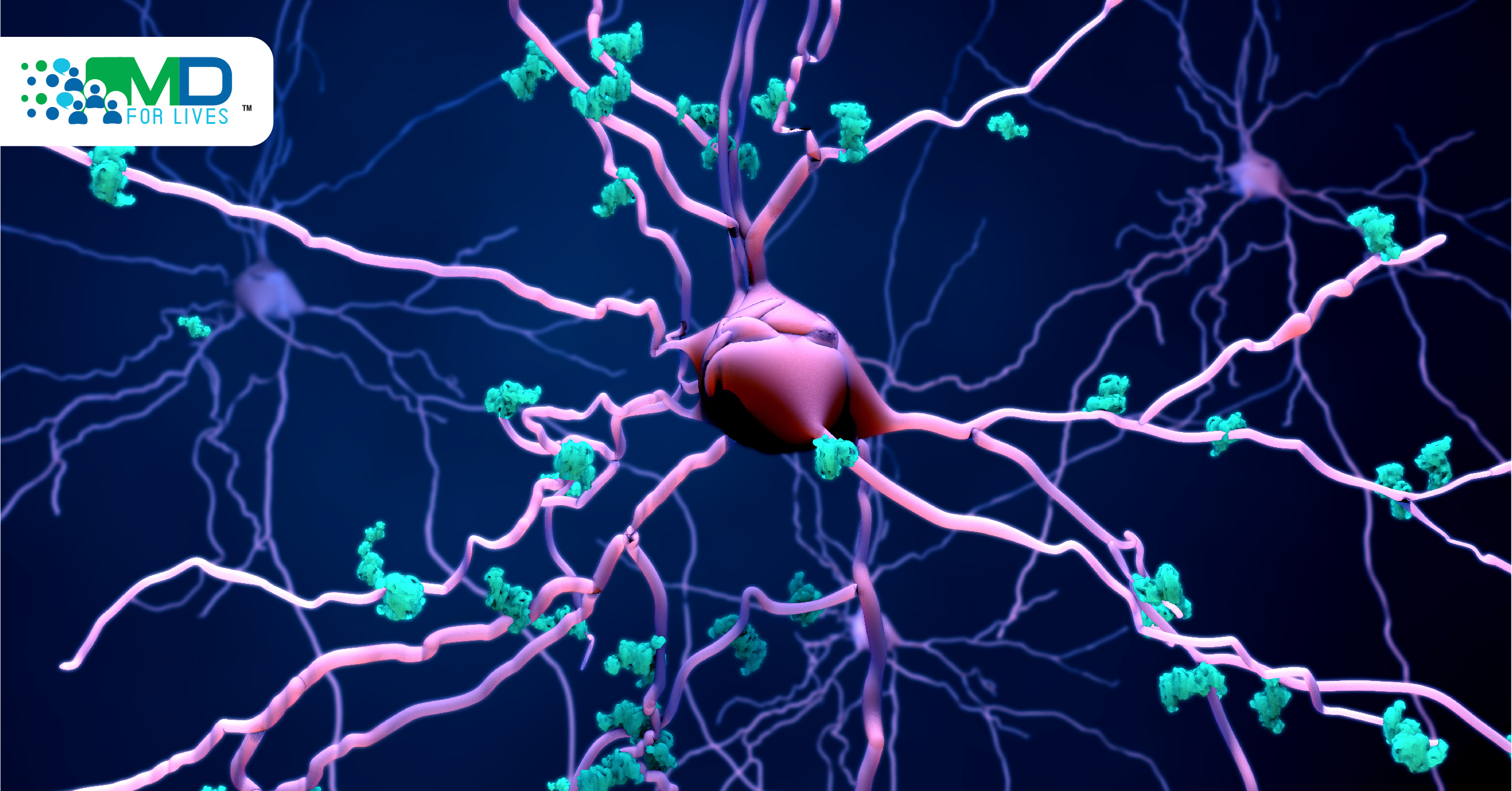
Alzheimer’s disease is a neurological disorder in which the brain shrinks and the brain cells involved in the memory die. In the United States, Alzheimer’s disease is the sixth leading cause of death. Toxic alterations occur in the brain when the disease is in the first stage, namely, the buildup of abnormal proteins that have the capacity to form amyloid plaques and tau tangles. The normally working neurons stop working, start losing connections to other neurons and eventually die. The two proteins that play role in Alzheimer’s are Tau and beta-amyloid.
A microtubule-associated protein known as “Tau” is present in the brain, which ensures the normal functioning of the brain cells. Beta-amyloid proteins are also present in the brain. In neuronal intracellular compartments, beta-amyloid peptides are generated and endosomes are the major site along with Golgi bodies responsible for the production of beta-amyloid.

Taupathy
Tau protein has been linked to Alzheimer’s disease and a variety of other debilitating brain diseases. Changes to the tau protein may influence the various ways it can misfold in a person’s brain cell and cause different types of neurodegenerative diseases. Neurodegenerative disorders that are caused by the deposition of abnormal tau protein in the brain are widely known as taupathies. Taupathies are of two types:
- Primary taupathy
- Secondary taupathy
Pick’s disease is considered under primary taupathy and Alzheimer’s under secondary taupathy.
Conducting Cryo-EM and mass spectrometry of tau filaments, it was found that post-translational modifications highly decorate Tau filaments and thus help in misfolding. Tau detaches from the microtubule and attaches to other Tau to form threads and then tangles form by joining of the threads.

Tau pathology
Tau pathology is more in autosomal dominant Alzheimer disease than late-onset Alzheimer disease. The difference between both groups could be due to various factors, namely, sporadic versus mutation, age of onset, or both. The hypothesis was tested on 10 patients enrolled at Knight Alzheimer Disease Research Center and 7 patients at the Dominantly Inherited Alzheimer Network. The patients were enrolled based on various criteria. Based on the burden of three parameters, including a neurofibrillary tangle, neuropil thread, and neuritic plaque distribution, pathology was determined. Neurofibrillary tangle varied significantly between the groups and the difference of mean neuritic plaque/neuropil thread between the two groups was also significant.

Spread of Tau
Huge clumps and tangles of misfolded proteins like tau replace the entire section of the brain. Through communication pathways of neurons, Tau transmits in the whole brain. In the brain’s interstitial fluid (ISF), beta-amyloid (Aβ) is present. In addition to tangles of Tau, plaques of beta-amyloid are an important component in Alzheimer’s disease patients’ brains. The spread of Tau in the entire brain is facilitated by the presence of β-amyloid (Aβ). Using an epidemic spreading model, Tau spread was tested. Tau spreads rapidly throughout the brain, as soon as the beta-amyloid reaches a tipping point. Other research in mice showed that sleep deprivation also causes Tau levels to rise and increase the chance of accumulation of the protein into harmful tangles. In the hippocampus and entorhinal cortex, Tau tangles become apparent and then get distributed in the whole brain. To test the hypothesis, the researchers tested two groups of mice. In the first group, the hippocampi of mice were introduced with Tau tangles and were kept awake for a long period of time, while in the second group, the mice introduced with Tau tangles were allowed to sleep. After 4 weeks, it was observed that in mice who were kept awake, Tau tangles had spread more than in the second group.

Measurement of Tau
Tau proteins can be measured in the blood with anti-Tau antibody. When a protein does damage in a living body, measurement of Tau levels can be useful. Tau levels in the fluid that surrounds the brain, both high and low, could be a warning sign. High levels of Tau indicate Alzheimer’s disease, whereas progressive supranuclear palsy is associated with low Tau levels. Transgenic mice were genetically modified with human tau. When the transgenic mice were administered with anti-Tau antibody, there was an increase in the concentration of Tau in the plasma of mice. When the same method was applied to human patients, there was also observed a similar result. However, the increase was dose-dependent. The half-life of Tau increased to 3 hours from a half-life of 8 min when administered with anti-Tau. The concentration of brain soluble and interstitial fluid tau decreased when insoluble tau accumulated in the brain of transgenic mice and in turn resulted in an increase of plasma Tau. This finding provides evidence that administration of anti-Tau antibody increases the concentration of plasma Tau, which corresponds to a decrease in the concentration of extracellular and soluble tau in the brain.



Alzheimer’s prevention
DNA is the building block for the production of proteins. DNA gets converted to proteins via the production of mRNA. Researchers have developed antisense oligonucleotides (ASOs) that bind to the mRNA and halt the production of protein. While building Tau, the genetic instruction required is targeted by the synthetic product and prevents the formation of Tau. The new finding has been shown to reverse tau-related damage to the brain. The hypothesis was tested in transgenic mice. Synthetic nucleotides could reduce Tau concentration in the brain of the mice and the mice lived longer. Antisense oligonucleotides get accumulated in the brain and spinal cord to reduce Tau concentration.

References:
https://www.nature.com/articles/s41467-020-15701-2
https://www.sciencedaily.com/releases/2020/02/200206144841.htm
https://www.science.org/lookup/doi/10.1126/science.aav2546
https://www.science.org/doi/10.1126/scitranslmed.aag0481
https://www.science.org/doi/10.1126/scitranslmed.aal2029
https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet
https://onlinelibrary.wiley.com/doi/abs/10.1111/tra.12808
https://onlinelibrary.wiley.com/doi/epdf/10.1111/nan.12208
https://www.science.org/doi/10.1126/scitranslmed.aal2029