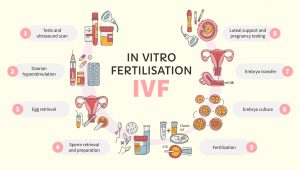
Since its clinical introduction in 1978, in vitro fertilization (IVF) has redefined the human species’ ability to reproduce. IVF was originally designed to help infertile couples, but clinical indications for IVF have rapidly expanded to include medical and genetic conditions, as well as fertility preservation. While access and utilization of IVF vary greatly across the globe, the practice now accounts for more than 5% of all newborns in some European countries where IVF is more affordable and/or covered by insurance.
Women’s improved access to educational and career opportunities, as well as effective contraception, has contributed to progressively delayed childbearing and overall lower fertility rates around the world. Fertility rates are now significantly lower than population replacement levels of 2100 births per 1000 women in many countries and virtually all US states. The average age at first birth now exceeds 30 years in a growing number of metropolitan areas and across entire highly developed countries, well beyond peak fertility, which occurs in the mid-20s.
Inadvertently, an increasing number of women are delaying childbearing to the point where age-related fertility decline contributes to the prevalence of infertility and increased demand for fertility treatments such as IVF and oocyte cryopreservation.
Controlled ovarian hyperstimulation (COH) is used to increase the number of available oocytes for IVF. COH entails multiple gonadotropin injections and multiple visits to the fertility clinic for transvaginal ultrasound evaluations and circulating hormone levels. Recent advancements in portable, low-cost ultrasound devices may further simplify follicular and endometrial monitoring through mobile facilities and possibly self-operated endovaginal telemonitoring. These approaches, when combined, have the potential to greatly simplify COH by making it less invasive and reducing the time commitment required. Finally, interventions that may reduce the treatment burden further include screening patients for psychological issues and providing counseling and coping interventions such as e-therapy as an integral part of IVF.
Recent studies suggest that gonadotropin stimulation and the oral medication letrozole used during

IVF cycles may be advantageous, particularly for breast cancer patients receiving fertility preservation therapy. To lower blood estrogen levels, letrozole and ovarian stimulation are used to treat breast cancer patients. According to these studies, compared to breast cancer-free controls who received conventional COH, breast cancer patients who received letrozole and gonadotropins for the duration of the stimulation had lower estradiol concentrations than they would have anticipated but also had more mature oocytes available for cryopreservation. Positive effects have been linked to it, such as increased oocyte and mature oocyte counts while maintaining the same pregnancy rate as traditional stimulation and decreased gonadotropin doses that reduce the cost of IVF therapy, and these effects have been linked to decreased gonadotropin doses.
However, gonadotropin stimulation can result in OHSS, severe hypoestrogenemia, and longer, more involved stimulation protocols. Letrozole’s effects on intraovarian testosterone levels and the success of IVF cycles were looked into in 2005. Garcia-Velasco et al. found that the use of letrozole 2.5 mg during the first five days of gonadotropin stimulation markedly increased the levels of androstenedione and testosterone in follicular fluid and enhanced the success of IVF cycles. Both the quantity of recovered oocytes and the implantation rate were significantly higher in the letrozole group than in the control group.
Because intraovarian androgens may have a significant impact on early follicular development, many methods have been studied to increase intrafollicular androgen concentrations in individuals who do not respond well to medication. Transdermal testosterone was used as a pre-treatment to improve the ovarian sensitivity to FSH and the follicular response to gonadotrophin therapy in low-responder IVF patients. The frequency of clinical pregnancies and live births increased along with the number of cumulus oocyte complexes. Gleicher et al. found that dehydroepiandrosterone (DHEA) was given as a supplement for 30 to 120 days to people with low ovarian reserve (25 mg three times per day). A prospective randomized controlled experiment was conducted by Wiser et al. to determine the impact of DHEA supplementation on the efficacy of IVF in patients with subpar responses. They found that compared to the controls, the DHEA group had significantly higher rates of live birth and higher-quality embryos. The quantity of zygotes and eggs was the same in both groups.
Microfluidics is defined as a multidisciplinary field of study and design in which fluid behaviors are precisely controlled and manipulated using small-scale geometric constraints that result in surface forces dominating volumetric counterparts. Despite their success, previous IVF laboratory procedures used approaches to microscale cellular biological events.
At least four benefits are foreseen from the incorporation of microfluidics into the IVF laboratory:
- precisely controlled fluidic manipulations of gametes and embryos;
- provision of biomimetic environments for culture;
- facilitation of microscale genetic and molecular bioassays; and
- enabling miniaturization and automation.
The use of time-lapse incubators, which enable continuous monitoring of embryo development, has already fully automated the culture of embryos. Machine learning can be used to analyze data from time-lapse incubators to help choose the embryos with the best chance of becoming pregnant.
Sperm, oocytes, and embryos are now routinely preserved using cryotechnology. The most popular technique for oocyte and embryo cryopreservation is vitrification.
Subcellular oocyte modification has been used in other programs to fight ooplasmic aging. Cohen et al. used donor oocytes to perform ooplasmic transfers into mature oocytes of patients whose numerous IVF attempts had failed due to inadequate embryo development. The results showed that the babies were healthy and alive. Because mitochondria are found in the cytoplasm, the fact that recipient eggs contained mitochondria from the donor ooplasm was thought to be the main factor influencing better development. Since testing for heteroplasmy on several healthy children revealed that their mitochondrial DNA (mtDNA) was derived from both the mother and the cytoplasm donor, proving that the heteroplasmy was present in the oocytes, ooplasm donation is no longer practiced.
The earlier work with ooplasm transfer has been improved by later research using autologous mitochondrial transfer. This state-of-the-art procedure involves removing the mitochondria from the oocyte precursor cells in the superficial epithelial layer of the patient’s ovary and injecting them into their oocytes during fertilization. It has been shown that mitochondrial injection improves embryo growth and helps women who previously had poor embryo development give birth to live babies.
- The Future of IVF: The New Normal in Human Reproduction. Springer Link. https://link.springer.com/article/10.1007/s43032-021-00829-3. Accessed on: 11.28.2022
- Recent Advancements in In Vitro Fertilisation. Cureus. https://www.cureus.com/articles/116877-recent-advancements-in-in-vitro-fertilisation. Accessed on: 11.28.2022

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.