Dermatomyositis (DM) is a rare autoimmune disease that can occur at any age but is most common among people aged 40 to 60. The disease is also more common among women. DM can lead to progressive muscle weakness and eventual disability. In many cases, patients initially develop debilitating skin changes on the face and body. In severe cases, the disease can affect other organ systems as well and can be life-threatening.
A study published on October 6, 2022, reports on a trial of intravenous immunoglobulin (IVIG) in the treatment of dermatomyositis. The trial found a higher rate of response in patients given treatment compared to placebo, but some patients experienced adverse events thought to be related to the IVIG treatment.
What is Dermatomyositis (DM)?
DM is an autoimmune disorder characterized by progressive muscle weakness. The specific underlying cause of DM is unknown. Patients with DM may exhibit varying symptoms and physical findings, but the major symptom is muscle weakness affecting the trunk and proximal muscles such as the hips, thighs, shoulders, upper arms, and neck.1 The onset of muscle weakness and skin rashes are caused by vasculitis, or the inflammation in the blood vessels within the skin and muscles.
Eventually, these symptoms lead to muscle degeneration and atrophy, which can affect daily functions. Patients may experience difficulties in performing tasks such as lifting their arms, climbing stairs, raising their head from the pillow when lying down, or getting up from a fall. In some cases, patients may experience dysphagia and dysphonia if muscles in the neck, tongue, or throat are affected.
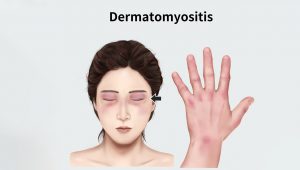
What distinguishes DM from other forms of myositis is the characteristic skin rashes that can co-occur with or precede the development of muscle weakness. These skin changes can include distinctive “heliotrope” rashes (reddish-purple or lilac) and “butterfly rash” on the face region, erythematous rashes (“dusky” reddish) on other parts of the body, and sometimes nail changes, edema, or hair loss.
The rashes tend to be itchy and can be extremely uncomfortable. Some patients, especially children, may develop hardened bumps under the skin (calcinosis) associated with contractures and atrophy of affected muscles and tissues.
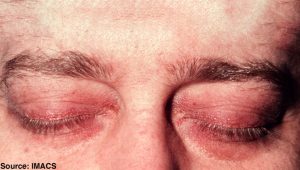

Caption: Characteristic skin rashes develop on the face and body of DM patients.
Current Treatments for Dermatomyositis
High-dose glucocorticoids are the first-line treatment to reduce inflammation in the skin and muscles. To reduce the adverse effects of glucocorticoids, such as bone density loss, the treatment is tapered and followed by other immunosuppressive agents such as methotrexate, azathioprine, or mycophenolate mofetil. However, therapy with harsh immunosuppressive agents can increase susceptibility to infections and can have other adverse effects.
In cases in which patients do not respond to the above treatments, IVIG with immunoglobulins extracted from human plasma has been used as an off-label and add-on therapy. Previous small clinical trials and other noncontrolled trials have suggested IVIG can be effective. However, IVIG has been associated with thromboembolic events.

Caption: Previous small clinical trials and other noncontrolled trials have suggested IVIG for dermatomyositis can be effective for those who do not respond to glucocorticoids or immunosuppressive agents.
A Clinical Trial of IVIG in Dermatomyositis
A phase III, multicenter, double-blind, placebo-controlled, randomized trial (“ProDERM study”, NCT02728752) was conducted to evaluate the long-term efficacy and safety of IVIG in DM patients. Randomization was stratified based on disease severity before the start of the trial. In the trial, 95 adults with DM received either an intravenous infusion of IVIG (2g/kg of body weight) or a placebo every 4 weeks for a total period of 16 weeks. Ninety-six percent of patients in both groups completed the 16-week study.
This was followed by an open-label extension phase for another 24 weeks; 72% of the IVIG-treated patients and 73% of placebo-treated patients participated. Ninety-nine percent of the patients also received either glucocorticoids or other medications.
The primary endpoint of the study was defined based on ‘at least minimum improvement,’ with a score of at least 20 at week 16 in the a Total Improvement Score (TIS), based on the 2015 American College of Rheumatology-European League Against Rheumatism (ACR-EULAR) myositis response criteria. The secondary efficacy endpoints included a response indicating ‘at least moderate improvement’ (TIS ≥40) or ‘major improvement’ (TIS ≥60) at week 16.
Results from the ProDERM Study:
At week 16, the primary endpoint was met, with 79% of the IVIG group and 44% of the placebo group showing TIS ≥20. In addition, a higher percentage of the IVIG group showed TIS ≥40 and TIS ≥60 (secondary endpoints). In the open-label extension phase of the study, the placebo patients that switched to the IVIG regimen showed an increased TIS level similar to that of the IVIG group by week 40.
Adverse events occurred mostly during the 72 hours after IVIG infusion and were higher in the IVIG treatment group. Five IVIG-treated patients developed serious adverse events including sepsis, pulmonary embolism, muscle spasm and dyspnea, and ventricular extrasystoles. In addition, 8 thromboembolic events were reported, with 6 of those occurring in the IVIG-treated group. Other adverse events included headache, pyrexia, and nausea.
Although the trial provides efficacy data for IVIG in DM, the authors conclude that larger and longer trials are needed to determine the long-term effects and risks of IVIG. The clinical trial received funding from Octapharma Pharamzaeutika and the Czech Ministry of Health. The current results from the ProDERM trial lead to the approval of an IVIG preparation for DM by the FDA, Health Canada, and multiple European regulatory agencies.

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.