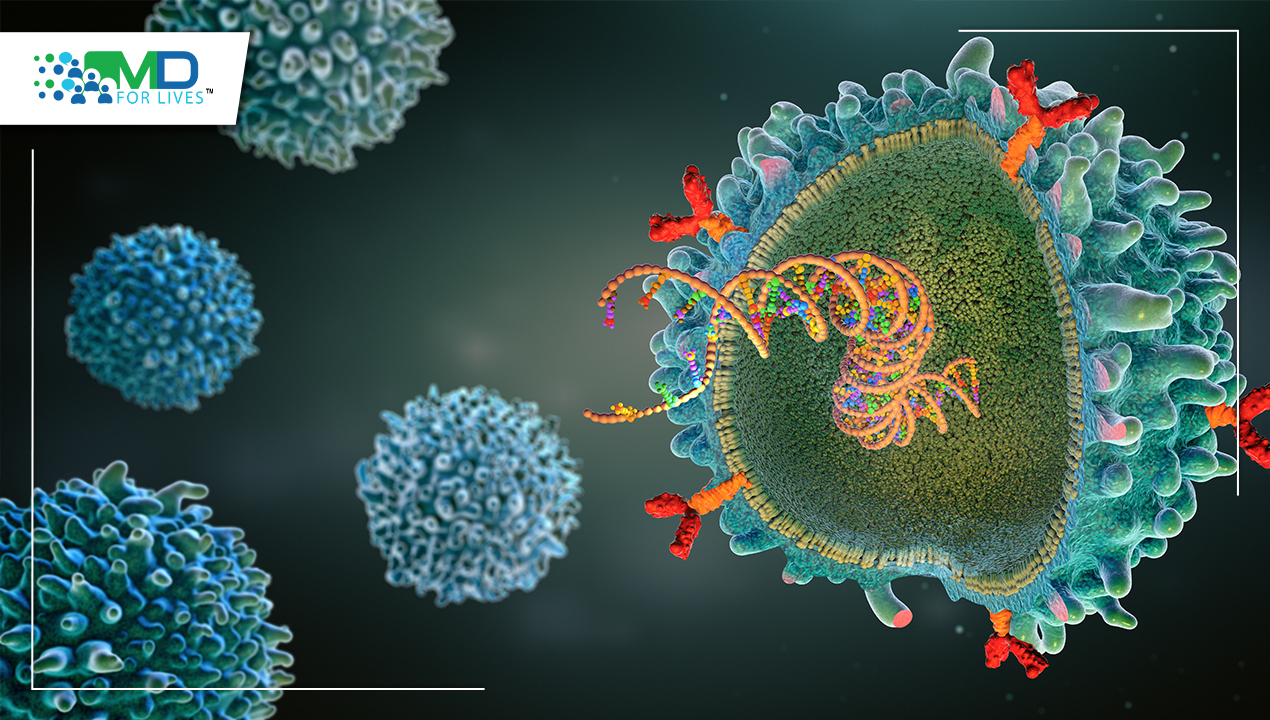
McMaster University, the University of Chicago, the Pasteur Institute, and other institutes studied and uncovered genes that protected certain people from the horrific bubonic plague epidemic that swept through Europe, Asia, and Africa approximately 700 years ago. The research was published in the journal Nature. According to the researchers, the same genes that formerly gave protection against the Black Death are now connected with an increased vulnerability to autoimmune disorders such as Crohn’s and rheumatoid arthritis. Key genetic distinctions that decided who survived and who perished during the Black Death epidemic have been uncovered by an international team of scientists who examined centuries-old DNA from victims and survivors. They have also shown how those immune system components have changed over time.
The Black Death was a pandemic that swept Europe, killing more people than any other known illness or warfare at the time. Western Eurasia and North Africa had a bubonic plague epidemic. It is the deadliest epidemic in recorded history, killing 75-200 million people and peaked in Europe from 1347 to 1351. Bubonic plague is caused by the bacteria Yersinia pestis, which is carried by fleas, but it may also be transferred through person-to-person contact via aerosols, resulting in septicaemic or pneumonic plagues. The Black Death marked the start of the second plague pandemic. The pandemic caused religious, social, and economic changes that had far-reaching consequences for European history.
The researchers concentrated on a 100-year period before, during, and after the Black Death, which struck London in the mid-1300s. It is still the single biggest human mortality incident in recorded history, killing up to 50% of the inhabitants in some of the world’s most densely inhabited areas. More than 500 ancient DNA samples were recovered and processed from the bones of those who died before, during, or after the Black Death in London, including those interred in the East Smithfield plague trenches used for mass burials in 1348-9. Additional samples were collected from bodies buried at five different places around Denmark. Scientists looked for evidence of genetic adaptation to the plague, which is caused by the bacteria Yersinia pestis. They identified four under-selection genes, all of which are involved in the creation of proteins that protect human systems from invading pathogens, and discovered that alleles of those genes either protected or rendered one susceptible to plague. Individuals having two identical copies of a certain gene known as ERAP2 survived the pandemic at a considerably greater rate than those with the opposite pair of copies, since the ‘good’ copies allowed immune cells to more efficiently neutralise Y. pestis.
When a pandemic of this magnitude happens, which kills 30 to 50% of the population, there is sure to be selection for protective genes in humans, which means those who are susceptible to the circulating disease will perish. Even a tiny edge might make the difference between survival and death. Those individuals who are breeding age will, of course, pass on their genes. Hendrik Poinar, author of the Nature research, head of McMaster’s Ancient DNA Centre, and principal investigator of the Michael G. DeGroote Institute for Infectious Disease Research and McMaster’s Global Nexus for Pandemics & Biological Threats, explains. Since they had not recently been exposed to Yersinia pestis, Europeans living during the time of the Black Death were at first extremely susceptible. Mortality rates fell as the epidemic recurred often throughout the ensuing centuries. According to research, individuals who had the protective allele for the ERAP2 gene (the healthy copy of the gene, or characteristic), had a 40–50%– higher chance of surviving than those who did not. Human geneticist Luis Barreiro, a co-author on the paper and professor of Genetic Medicine at the University of Chicago, said that the selective advantage connected to the chosen loci is among the strongest ever reported in humans and demonstrates how a single pathogen can have such a significant impact on the evolution of the immune system.
According to the research team, throughout our immune systems have changed how they react to infections, to the point that a gene that in the Middle Ages protected against plague is now linked to a higher risk of developing autoimmune illnesses. This is the delicate balancing act that evolution has performed with our genome. According to Javier Pizarro-Cerda, head of the Yersinia Research Unit and director of the World Health Organization Collaborating Centre for Plague at the Pasteur Institute, this highly original work has only been made possible through a successful collaboration between very complementary teams working on ancient DNA, on human population genetics, and on the interaction between live, virulent Yersinia pestis and immune cells.
The research, which took seven years to complete and was led by postdoctoral researcher Tauras Vigylas of the University of Chicago and graduate student Jennifer Klunk, formerly of McMaster’s Ancient DNA Centre, allowed for a previously unheard-of examination of the immune genes of Black Death victims.
Reference:
- https://www.nature.com/articles/s41586-022-05349-x
- https://www.britannica.com/event/Black-Death
- https://en.wikipedia.org/wiki/Black_Death

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.