Non-Hodgkin lymphoma treatment traditionally relied on chemotherapy, radiation, and bone marrow transplant. More recently, immuno-oncology treatments have come to the forefront.
In February, the FDA approved an immunologic gene therapy known as CAR-T therapy for diffuse large B-cell lymphoma, a type of non-Hodgkin lymphoma.1 The new therapy is called Breyanzi (lisocabtagene maraleucel) and is marketed by Juno Therapeutics, part of Bristol-Myers-Squibb.
What is CAR-T cell therapy?
CAR-T cell therapy is a type of gene therapy involving the patient’s T cells, which are genetically manipulated outside the body. Several CAR-T cell-based therapies have been approved in different types of cancer.2
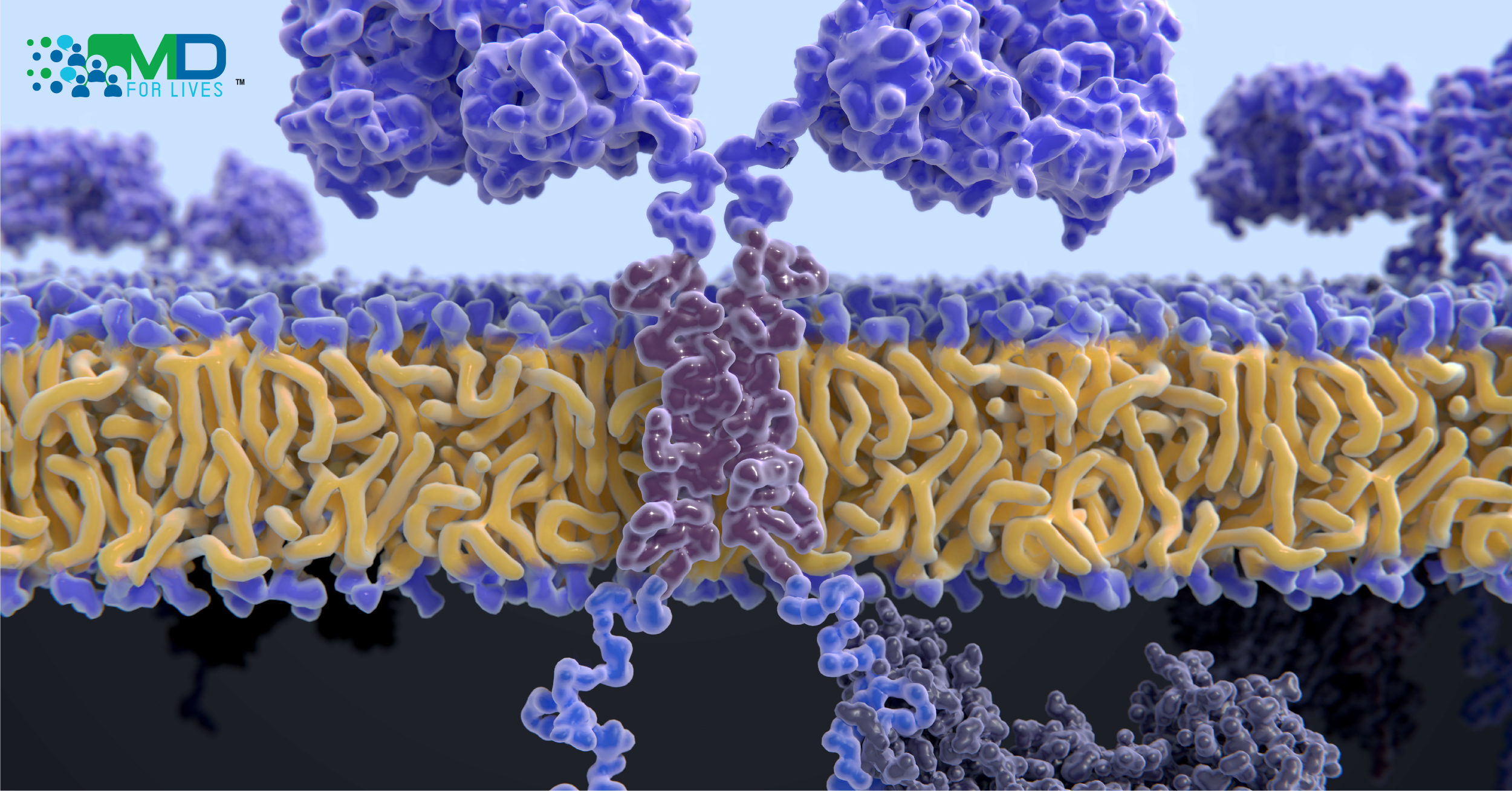
Chimeric antigen receptor T cells, referred to as CAR-T cells, are genetically modified to produce an engineered protein called a chimeric antigen receptor. This gives them an enhanced ability to locate and destroy cancer cells that express a specific, targeted antigen.2

The CAR-T cell therapy process involves collecting T cells from the patient’s blood via leukapheresis. In a laboratory, a gene coding for a chimeric antigen receptor (CAR) is inserted into the T cells. Chemotherapy is typically given to deplete the patients’ unmodified T cells before the modified CAR-T cells are infused. Once the modified cells are returned to the body, the CAR protein expressed on the T cell’s surface causes it to bind to a specific population of cells- the cancer cells that express the targeted antigen. Simultaneously, the intracellular portion of the CAR stimulates the T cell to attack.2,3
Conceptually, the CAR-T strategy is similar to the one used by Amgen’s BiTE products, in which recombinant bispecific antibody-like proteins are used to physically link T cells to cancer cells and facilitate an attack. In the case of CAR-T, the T cells themselves are modified to produce a receptor that binds the antigen.2
The CAR used in Breyanzi targets CD19, an antigen found on B cells. Breyanzi marks the fourth CAR-T therapy, approved by FDA.3 The previously approved tisagenlecleucel (Kymriah), brexucabtagene autoleucel (Tecartus), and axicabtagene ciloleucel (Yescarta) also target the CD19 antigen. On March 27, 2021, the FDA approved Abecma (idecabtagene vicleucel) as a fifth-line treatment for multiple myeloma, which targets the B-cell maturation antigen (BCMA) present on multiple myeloma cells.4

Other antigens are being targeted in investigational products, including CD22, Lewis Y, and CD20.2 Ide-cel (decabtagene vicleucel) is an investigational CAR-T therapy that targets BCMA. Results from a Phase 2 study were reported on February 25, 2021, in the New England Journal of Medicine. The treatment showed promise in patients with heavily treated, relapsed multiple myeloma, with 33% of the 128 treated patients achieving a complete response.5
Challenges and promise of CAR-T cell therapy
Like BiTEs, CAR-T treatment strategies have found success against hematologic malignancies, but there have been challenges in directing them against solid tumors.2 Also, because the patient’s cells are used, each CAR-T treatment is unique and can only be used for a single patient. This and the need for hospitalization have led to very high costs for the treatments.6
Toxicity is also a major issue but has been considered acceptable based on risk-benefit considerations for appropriate patients.2 Hematologic toxicity, neurotoxicity, infections, and cytokine release syndrome can occur with various CAR-T treatments, and some of these events have been fatal.2,5
CAR-T cells can replicate in the body after infusion, and in some patients, the modified cells can persist in the blood for months or over a year after a single dose. Researchers are working on improving the persistence of future CAR-T cell treatments, which could support sustained efficacy.5,7
Breyanzi clinical trials and approval
The recently approved CAR-T cell therapy Breyanzi was investigated in the TRANSCEND NHL 001 trial.8 Of 269 study participants with relapsed or refractory large B-cell lymphomas who were treated with Breyanzi, 256 patients were included in the efficacy evaluation. An objective response was observed in 186 patients (73%, 95% CI 66.8-78.0%) and a complete response was observed in 136 patients (53%, 95% CI 46.8-59.4%) Adverse events including cytokine release syndrome, neurological events, and dose-limiting toxicity were reported during the study. One patient died from effects though to be related to the treatment.8

Breyanzi is approved for use in diffuse large B-cell lymphoma, high-grade B cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B. It is not approved for primary central nervous system lymphoma. The treatment is indicated for adult patients who have previously been treated with at least two other types of systemic treatment but have relapsed or have not responded.1
The FDA approval includes a boxed warning regarding cytokine release syndrome and neurologic toxicities, and a risk evaluation and mitigation strategy (REMS) program is required. The product will be administered at treatment centers certified in the REMS.1