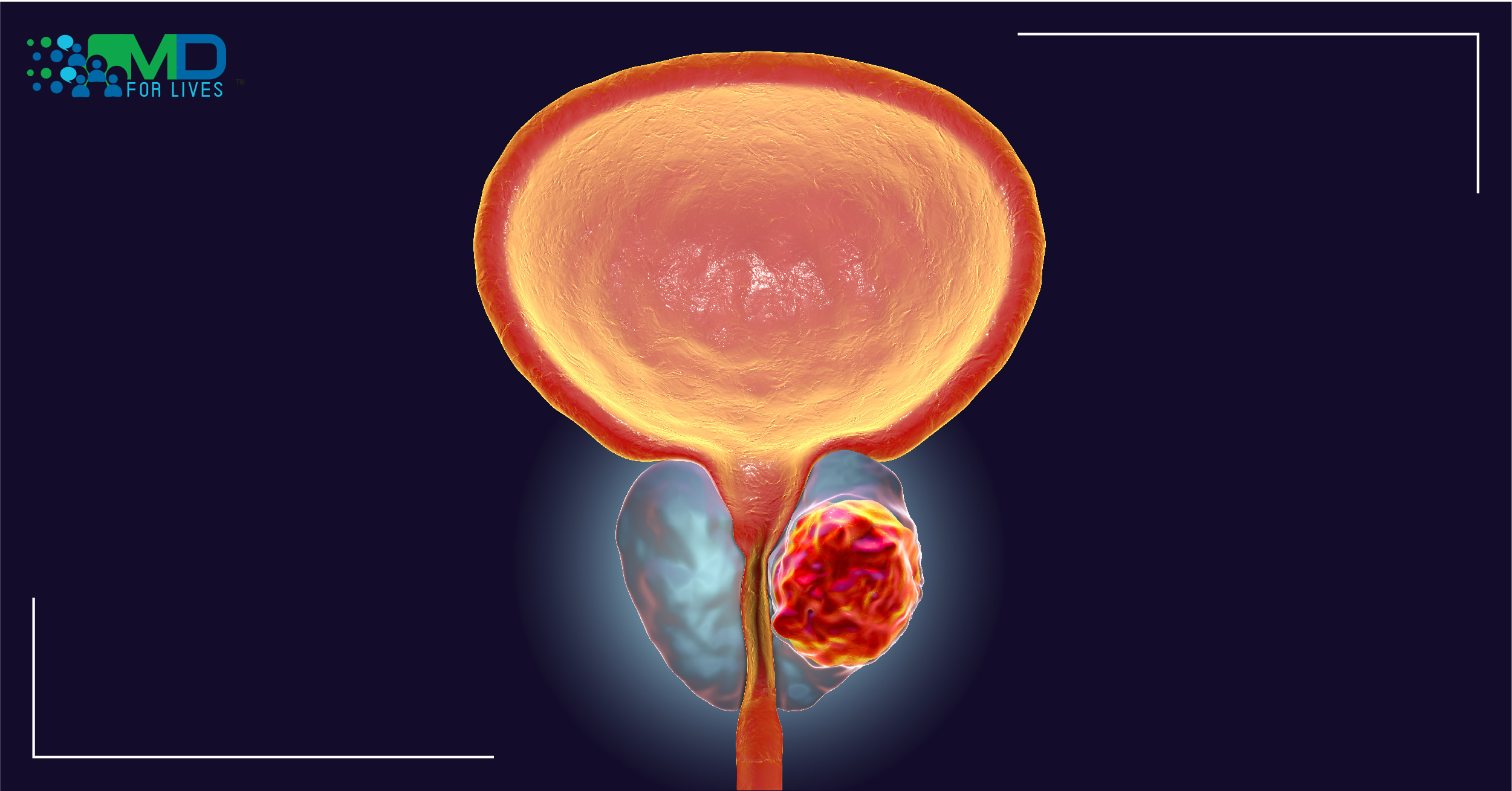
Prostate cancer is one of the most common cancers and a second leading cause of cancer death in American men.
Androgens stimulate prostate cancer. Androgen receptor, a ligand-dependent transcription factor is critical for prostate cancer development and progression. As a first-line treatment for prostate cancer, hormonal therapies target the androgen receptor. The initial response for androgen depletion and AR-targeted therapies are generally favorable; however, most tumors eventually resist primary hormone therapy and progress to recurrent castration-resistant prostate cancer (CRPC).
The current study solves the puzzle of why the androgen-targeting treatments are ineffective at later stages.
Circadian Rhythm and Cancer
Circadian disruption has been identified as an independent risk factor of various cancers including colon, breast, and prostate cancers. The biological or circadian clock, the natural timing device regulates and synchronizes bodily functions such as the sleep-wake cycle, hormone secretion, etc., rhythmically with the day/night cycle. Disruption in the biological clock can affect the physiology of the body, affects communication between cells and tissues, resulting in subsequent deregulation- associated with the development and progression of various diseases including cancer.
Several epidemiological studies have shown an association between increased incidence of cancer and disrupted circadian rhythms due to night shift; however, the underlying mechanism was not very clear.
Recently researchers investigated the link between the circadian clock and cancer hallmark pathway genes. The participants underwent 3 days of either simulated night-work schedule or simulated day-work schedule. Followed by a 24-hour constant routine protocol during which circulating leukocytes were obtained from the participants. Those who had been in the simulated night shift showed significant alterations in the circadian rhythm of cancer hallmark pathway genes. The DNA repair rhythmicity was enhanced in the day-shift schedule but the rhythmicity was lost in the night-shift schedule. The findings indicate that night shift schedules can disrupt the activity of certain cancer-related genes, alters the rhythmicity in DNA repair genes. The circadian dysregulation of DNA elevates DNA damage and potentially increases the risk of cancer in night shift workers.
Circadian Factor CRY1 and Prostate Cancer
Loss of circadian rhythmicity is also linked to poor anticancer treatment outcomes. In a recent study, researchers explored the circadian clock in prostate cancer and discovered that the transcriptional coregulator of circadian rhythm CRY-1 (cryptochrome circadian regulator 1) promotes tumor progression in prostate cancer by altering DNA repair.
Researchers analyzed the human cancer data and found elevated circadian factor CRY-1 in late-stage prostate cancers is strongly associated with poor outcomes; however, the role of CRY-1 was not very clear. The urgent need to develop new therapeutic strategies for prostate cancer led researchers to explore the circadian clock. The study uncovered the role of the CRY-1 gene in prostate cancer progression.
Cancer treatment disrupts the cancer cell’s DNA repair mechanism and induces cancer cell apoptosis. In cultured cells, animal models, and tissue harvested from prostate cancer patients, researchers investigated the role of CRY-1 in the DNA repair process. They found CRY-1 is androgen-regulated and gets elevated when radiation induces DNA damage in cancer cells. They also found CRY-1 regulates the expression of crucial factors required for the DNA repair mechanism and affects the efficacy of the treatment. The CRY1 cistrome and transcriptome mapping reveal that CRY-1 is a pro-tumorigenic factor that modulates DNA repair mechanism, promotes DNA repair, and plays a protective role against DNA damaging cancer treatment.
The elevated level of androgen-responsive CRY-1 in late-stage prostate cancer explains why androgen receptor targeted therapy, the commonly used hormone therapy for prostate cancer becomes ineffective at the later stage of prostate cancer.
The collective findings shed light on the unexpected role of CRY1 in metastasis and poor outcomes in prostate cancer. The study paves the way for targeted treatment in advanced prostate cancer. The genome-wide insight into CRY1 function in prostate cancer opens new opportunities for researchers to explore therapeutic strategies to target and block CRY-1 and inhibit DNA repair in prostate cancer cells.

https://onlinelibrary.wiley.com/doi/10.1111/jpi.12726
https://www.nature.com/articles/s41467-020-20513-5