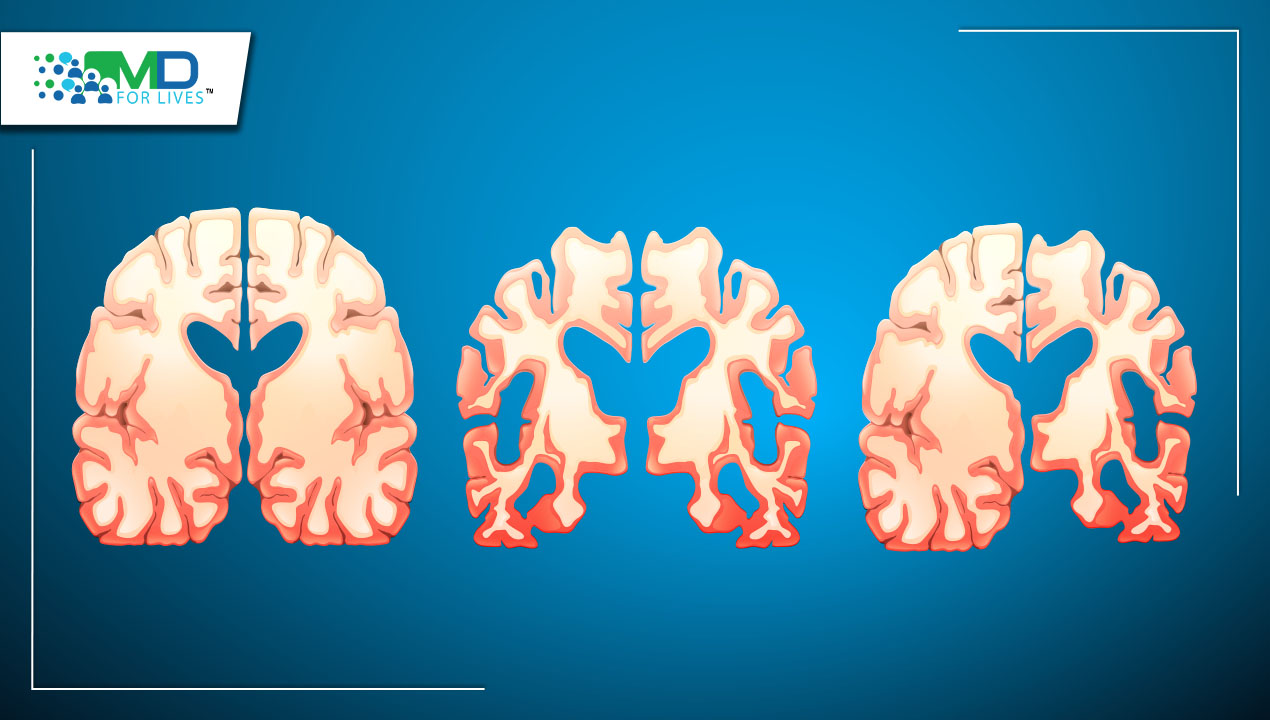
Alzheimer’s disease (AD) is characterized by pathologically accumulating amyloid plaques and neurofibrillary tau tangles in the brain. The systematic diversity in the distribution of AD tau pathology results in a wide variety of diseases.
A recent article that covers significant debates in decoding the complexity of AD subtypes, explores potential reasons for AD variability in the population. Additionally, discusses the clinical implications and future directions of research into AD heterogeneity that can be helpful to medical professionals.
What are the different clinical subtypes of Alzheimer’s Disease?
Typically, Alzheimer’s Disease has been linked to a late-life syndrome that steadily progresses from memory loss to multidomain cognitive impairment to full dementia. Although Alzheimer’s Disease is the main disorder, numerous syndromes have been identified that first and predominately affect either visuospatial cognition, language production, executive function, personality, and behavior or motor faculties.1 These non-amnestic AD patients frequently manifest earlier than amnestic AD cases, frequently before the age of 65.
Radiological presentations of AD variants
The gradual cognitive decline of late-onset amnestic AD is followed by progressive neurodegeneration, primarily of medial temporal lobe structures and, subsequently, posterior transmodal association cortex. While the hippocampus and medial temporal structures vulnerable in amnestic AD are typically relatively spared in young-onset and non-amnestic clinical presentations of AD, which frequently display tau accumulation and neurodegeneration in regions extremely specific to the domain impaired.
‘Typical’ and ‘Atypical’ AD
Many researchers have termed these syndromes “atypical AD” in order to contrast them with “typical” late-onset amnestic presentations. This is due to the identification of Alzheimer’s Disease variations with an (often) early age of onset and substantial initial deficits in nonmemory areas.
In an autopsy investigation of AD patients using a multisite dataset of more than 2000 tau-positron emission tomography images, researchers discovered systematic variation in the relative geographical distribution of tau tangles. They also documented the existence of a posterior and an asymmetric lateral temporal pattern of tau accumulation.
They did not discover a single dominating tau pattern in their sample that corresponded to the definition of a “typical” AD. Instead, memory impairment was present in all four AD tau subtypes, and ‘typical’ atrophy patterns appeared at various times along each subtype’s trajectory
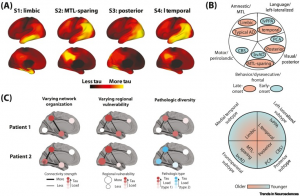
Fig. 1: Conceptualizing the classification and potential source of variation in AD tau pathology
The extremes of a spectrum or unique entities: what are atypical phenotypes?
Despite being present in both early-onset and late-onset cases, several of the tau subtypes listed earlier have a lot in common with nonamnestic clinicoradiological disorders that have been previously described. In fact, there is a striking geographic association between the atrophy patterns of cognitively characterized late-onset subgroups and atypical clinical variations. It is currently unknown if these phenotypes represent two separate entities or two points on a continuum (Figure 1B). Nevertheless, the finding that tau can deposit preferentially in particular brain regions in some patients, regardless of the age at which it first manifests, suggests that different patient groups may share a degree of vulnerability. To better understand AD neurobiology, it may be crucial to look into subtypes of the disease based on biology (such as tau pathology) rather than clinical symptom expression.
Potential sources of variation in AD expression
Little is known about the causes behind the wide variations in regional expression, symptoms, and severity associated with AD. According to one study, biochemical traits, such as the location of tau phosphorylation, have a significant role in the diversity in the rate of tau seeding, correlate with the speed of cognitive decline, and have an inverse relationship with age.
Another possible cause of age-related variation in illness progression rate is population diversity in the elimination of pathogenic proteins.
The probable causes of AD subtypes
The following factors contribute to the diverse distribution of Alzheimer’s Disease subtypes:
- Variation in brain organization: The spread of pathology is initiated by a biochemical mechanism that is constant across people, but the epicentre changes due to significant population variances in brain architecture.
- Variation in regional vulnerability: Although the beginning pathogenic cascade may not differ between individuals, certain vulnerable brain regions may experience disproportionate burdens at different points in the cascade. A unique progression may then result from the prevalence of a pathological burden in one area over another.
- Pathologic diversity: The population may have different disease pathology biochemistry, and this variation may affect how the disease presents itself. Pathologic variety may vary due to previously unknown individual variances in enzyme activity, co-pathology, or other biological processes.
Future directions for research on AD subtype
Age of onset and AD subtype are also important factors in the rate of longitudinal decline, but further study is needed to understand how different AD symptoms can affect clinical management, prognosis, or treatment. To more fully understand the variations in AD expression, much work is still required. To do this, people across various AD subtypes would have their functional neuroimaging, cerebrospinal fluid, and multi-omic regional tissue profiles surveyed. Insofar as different AD subtypes represent diverse underlying disease biology, a priori or post hoc identification of subtypes may assist identify patient populations who respond better or worse to various therapies, hopefully resulting in more individualized treatment.
The accumulating evidence discussed here points to subgroups of Alzheimer’s Disease that are otherwise unique from other AD symptoms and represent internally consistent patterns of population variation. This observation is encouraging for the identification of the origins of this variation since it suggests that these sources may be quite frequent in the population and have detectable effect sizes. Therefore, researching subtypes could be a first step toward developing a customized AD treatment plan.
References:
- https://www.sciencedirect.com/science/article/abs/pii/S1474442220304403
- https://www.cell.com/trends/neurosciences/fulltext/S0166-2236(22)00033-9_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0166223622000339%3Fshowall%3Dtrue

MDForLives is a vibrant community of healthcare professionals and patients dedicated to shaping the future of healthcare. We provide valuable global insights to healthcare companies through online surveys, interviews, and discussion forums.