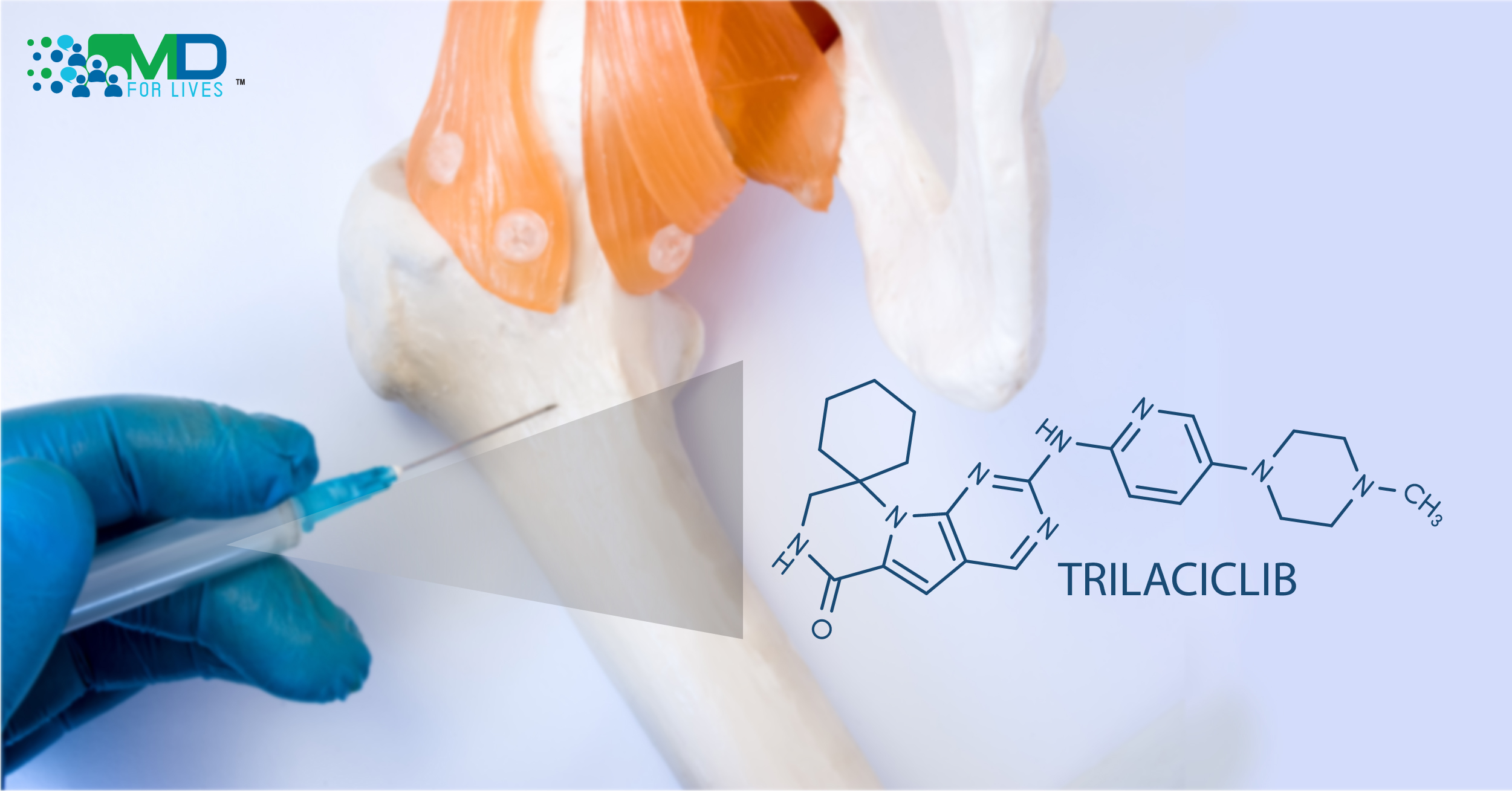
Chemotherapy can be lifesaving, but it can also damage bone marrow and cause blood cell counts to drop, sometimes to dangerously low levels. Now, the FDA has approved a new drug to reduce bone marrow suppression during the treatment of lung cancer.1
The drug, trilaciclib, is a first-in-class agent that showed good results in clinical trials with small-cell lung cancer patients.2 It is also under investigation for possible use in protecting patients’ bone marrow during treatment for other types of cancer.3
Chemotherapy and bone marrow suppression
Bone marrow suppression (myelosuppression or myelotoxicity) is a condition in which damage to the bone marrow prevents it from producing enough red blood cells (anemia), white blood cells (leukopenia), and platelets (thrombocytopenia).4 Bone marrow suppression is one of the most important side effects of cancer treatments.5
The association between chemotherapy and bone marrow suppression is well known and has severe consequences. Bone marrow suppression can lead to fatigue, shortness of breath, increased risk of infection, and increased risk of bleeding.1,5 Some patients require treatment dose reductions and delays, which can jeopardize successful treatment.1,5 Protecting the bone marrow during chemotherapy can result in enhanced patient quality of life and treatment efficacy.5

Current treatments for chemotherapy-induced bone marrow suppression
Until recently, supportive treatments like hematopoietic growth factors and transfusions were the only treatment options available for chemotherapy-induced bone marrow suppression.6,7 The disadvantages of these treatments are: they target specific blood cells only, they can be risky, and they are used after the chemotherapy has damaged the bone marrow.6,7
The ideal approach would be to protect the bone marrow before the damage is done.6,7 Trilaciclib showed promising results as a bone marrow protectant during chemotherapy treatment of patients with lung cancer.1

Trilaciclib mechanism of action
Trilaciclib acts by selectively, transiently, and reversibly inhibiting the cell-cycle enzymes cyclin-dependent kinases 4 and 6 (CDK4/6).8 CDK4/6 are needed for hematopoietic stem and progenitor cells to proliferate, and when these kinases are inhibited by trilaciclib, the cells remain in the G1 phase of the cell cycle and therefore are protected during chemotherapy that primarily kills replicating cells.6,8

Clinical trials and approval
Researchers tested the ability of trilaciclib to reduce chemotherapy-induced bone marrow suppression in several randomized, double-blind, placebo-controlled, phase II trials. Three of these studies were conducted in patients with extensive-stage small-cell lung cancer (ES-SCLC).6,7,9 Chemotherapy for SCLC is associated with severe bone marrow suppression, especially with second-line treatments.7
Before chemotherapy was initiated, patients received intravenous injections of trilaciclib or placebo.6,7,9 Between these three trials, 123 patients received trilaciclib and 119 received placebo, followed by chemotherapy treatments, etoposide/carboplatin [E/C], E/C/atezolizumab, or topotecan.2
The data from these three trials were pooled and the results were published in The Journal of Clinical Oncology in May 2020.2
The pooled data from these trials showed that trilaciclib reduced chemotherapy-induced bone marrow suppression without affecting the antitumor effects of chemotherapy.2 Patients who received trilaciclib were less likely to develop severe neutropenia (11.4% vs. 52.9%, p<0.0001) and the duration of severe neutropenia was shorter (0 days vs. 4 days, p<0.0001) compared to the placebo group.2 Fewer patients in the trilaciclib group needed chemotherapy dose reduction after cycle 1 of the treatment compared to the placebo group (9.2% vs. 30.8%).2
On February 12, 2021, the FDA approved the use of trilaciclib to protect the bone marrow during chemotherapy treatment of patients with ES-SCLC.1
Ongoing clinical trials are testing the benefits of trilaciclib in other cancers, including colorectal cancer.10 The safety and activity of trilaciclib during chemotherapy were also tested in patients with metastatic triple-negative breast cancer who received gemcitabine and carboplatin chemotherapy.3 While this phase 2 study did not show a significant difference in bone marrow suppression, the treatment was well-tolerated, and there was a possible positive trend in overall survival, a secondary endpoint.3

- https://www.fda.gov/news-events/press-announcements/fda-approves-drug-reduce-bone-marrow-suppression-caused-chemotherapy
- https://ascopubs.org/doi/abs/10.1200/JCO.2020.38.15_suppl.12096
- https://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(19)30616-3/fulltext