Discovering treatments for Duchenne muscular dystrophy has been difficult because the disease is a result of a wide variety of mutations in one of the human body’s longest genes. Sarepta Therapeutics has been working on targeting a series of Duchenne mutations using a single strategy – a type of drug called an exon-skipping therapy. The FDA has approved two of these drugs, eteplirsen and golodirsen in the past five years.1
Now, the FDA has approved Sarepta’s third exon-skipping drug, casimersen, despite criticism that the drug’s therapeutic benefit for patients is uncertain. The three drugs have also caused controversy because of their high price tags.1
What is Duchenne Muscular Dystrophy?
Duchenne muscular dystrophy (DMD) is a devastating muscle-wasting disease. Progressive muscle weakness eventually robs patients’ ability to walk, usually by the early teen years, followed by eventual failure of all voluntary muscles. Damage to the respiratory muscles and/or the heart most commonly leads to death in the third decade, though some patients live longer.
The disease results from one of a range of mutations in the gene for dystrophin, a key protein in the function and survival of muscle fibers. Lack of functional dystrophin damages muscle fibers and gradually causes their replacement with connective tissue and fat.
The disorder mainly affects boys because DMD is an X-linked recessive disease. Closely related is Becker muscular dystrophy, which is caused by milder mutations in the dystrophin gene.
Treatment for DMD
Treatment includes physical therapy and exercise to strengthen muscles; the use of braces and mechanical aids; and the use of beta-2 agonists, which have a short-term effect on muscle function. Corticosteroids are an important part of treatment and are considered to slow disease progression by several years. Patients need respiratory assistance in the later stages of the disease, and some patients need a pacemaker as well.
A drug called ataluren has been approved in the EU and Brazil as a targeted treatment for DMD, but the US FDA declined to approve it due to controversy over whether it offers a clinical benefit. Further studies are ongoing.
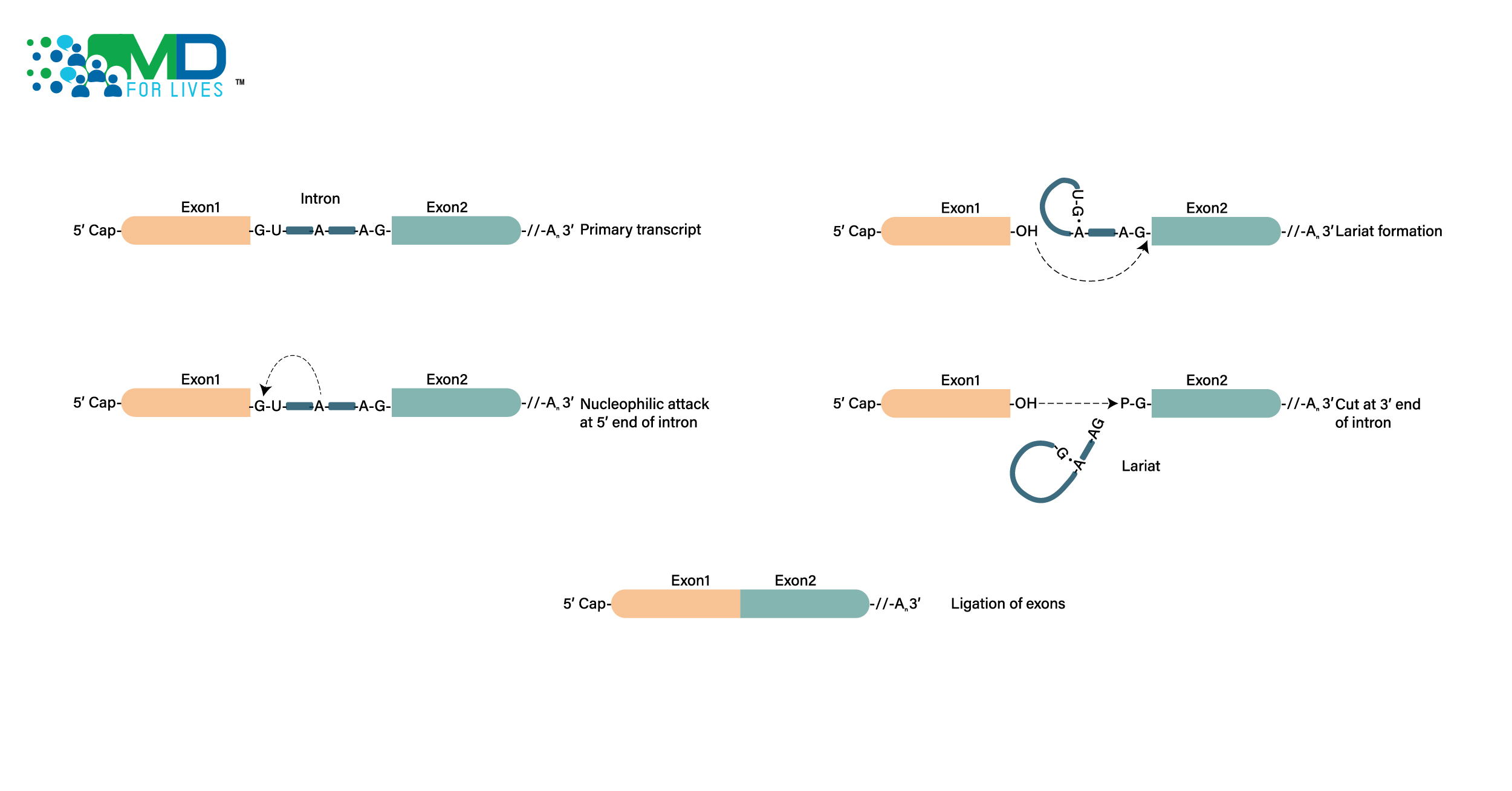
Four antisense drugs (DNA-like drugs that bind to mRNA and alter protein production) have been approved by FDA for different forms of DMD. All four are exon-skipping therapies.
DMD Exon Skipping Therapy
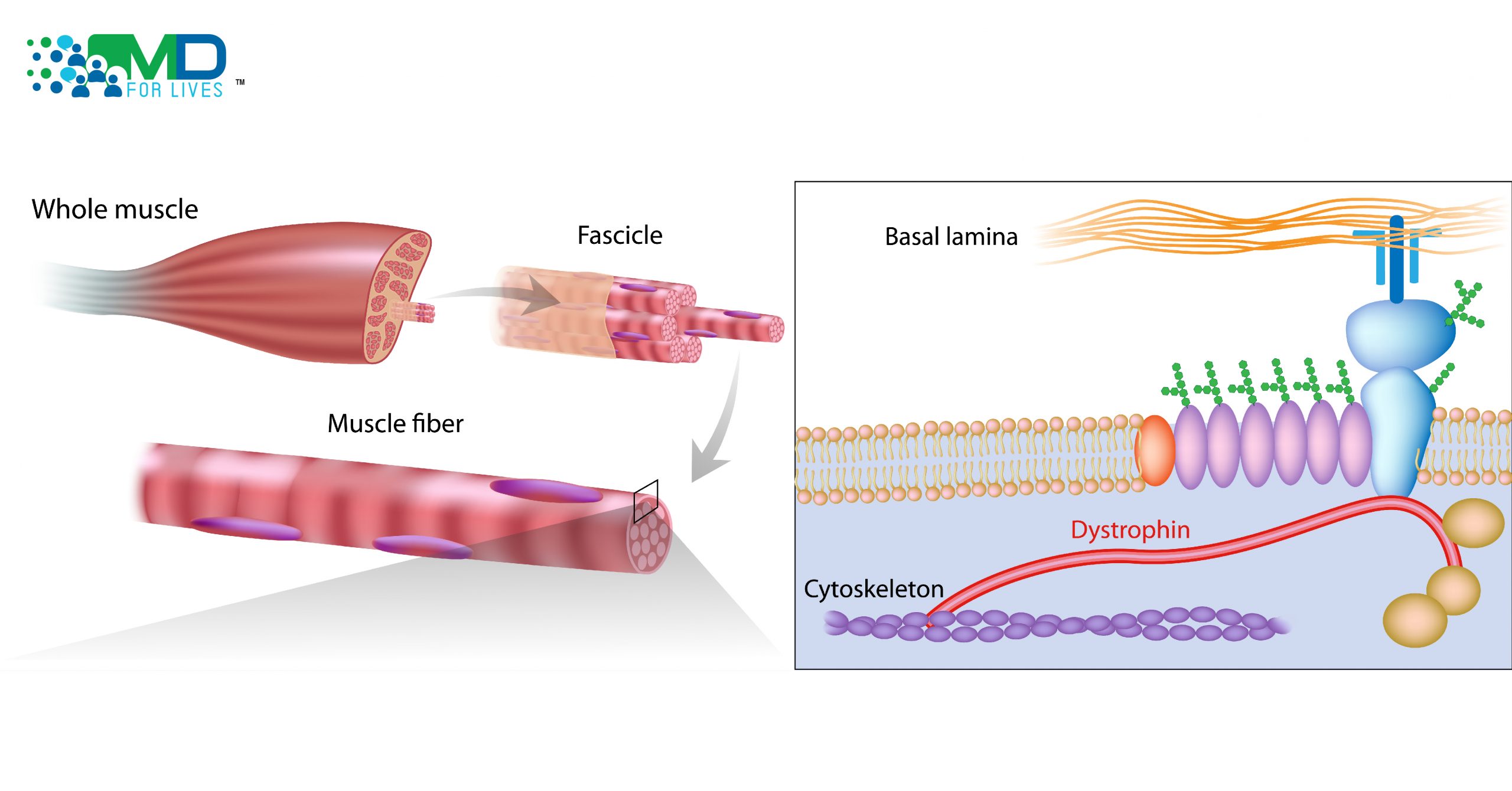 The gene mutations behind DMD lead to the production of a dysfunctional dystrophin protein that cannot fulfill its normal function. The concept behind exon-skipping therapy is that a faulty section of the gene can be skipped over, and thus left out of the final protein, but the remainder of the protein will be produced normally. This is thought to result in a shortened, but still partially functional version of the protein.
The gene mutations behind DMD lead to the production of a dysfunctional dystrophin protein that cannot fulfill its normal function. The concept behind exon-skipping therapy is that a faulty section of the gene can be skipped over, and thus left out of the final protein, but the remainder of the protein will be produced normally. This is thought to result in a shortened, but still partially functional version of the protein.
Dystrophin is a very large protein that helps anchor the cytoskeleton of muscle cells to the extracellular matrix and also has roles in cell signaling. The protein is crucial for muscle fiber function and survival.
In 2016, Sarepta Therapeutics’ antisense drug eteplirsen became the first exon-skipping therapy approved by the FDA. Marketed as Exondys 51, it is designed for patients with mutations in dystrophin exon 51, and a clinical trial showed the drug leads to the production of a shortened form of dystrophin in patients’ muscles.
Next, the FDA approved Vyondys 53 (golodirsen) in 2019, despite the agency’s rejection of the same therapy just a few months earlier. At the time of the rejection, the FDA cited an infection risk and a kidney toxicity issue seen in animal studies. The FDA approved the antisense drug Viltepso (viltolarsen), made by NS Pharma, for use in patients with the same group of mutations, in August 2020.
The latest FDA approval, on February 25, 2021, is for Amondys 45 (casimersen), an exon 45 skipping antisense drug. The ESSENCE trial of casimersen showed that patients could produce a small amount of the truncated protein after treatment, meeting the trial’s primary biological endpoint.
However, the trial failed to show a statistically significant improvement in functional motor abilities in treated patients. The trial was also small; a total of 43 patients in the trial were randomized 2:1 to receive casimersen or placebo.
All three of Sarepta’s therapies have been controversial. The European Medicines Agency (EMA) has declined to approve eteplirsen, citing weak efficacy data and the small size of the clinical trial.
Meanwhile, the drugs’ very high price has caused concern, especially in light of the uncertain clinical benefit. The FDA is requiring Sarepta to conduct confirmatory trials to provide further evidence of efficacy for all three products. Results from the confirmatory trial for casimersen are expected in 2024.
Also Read