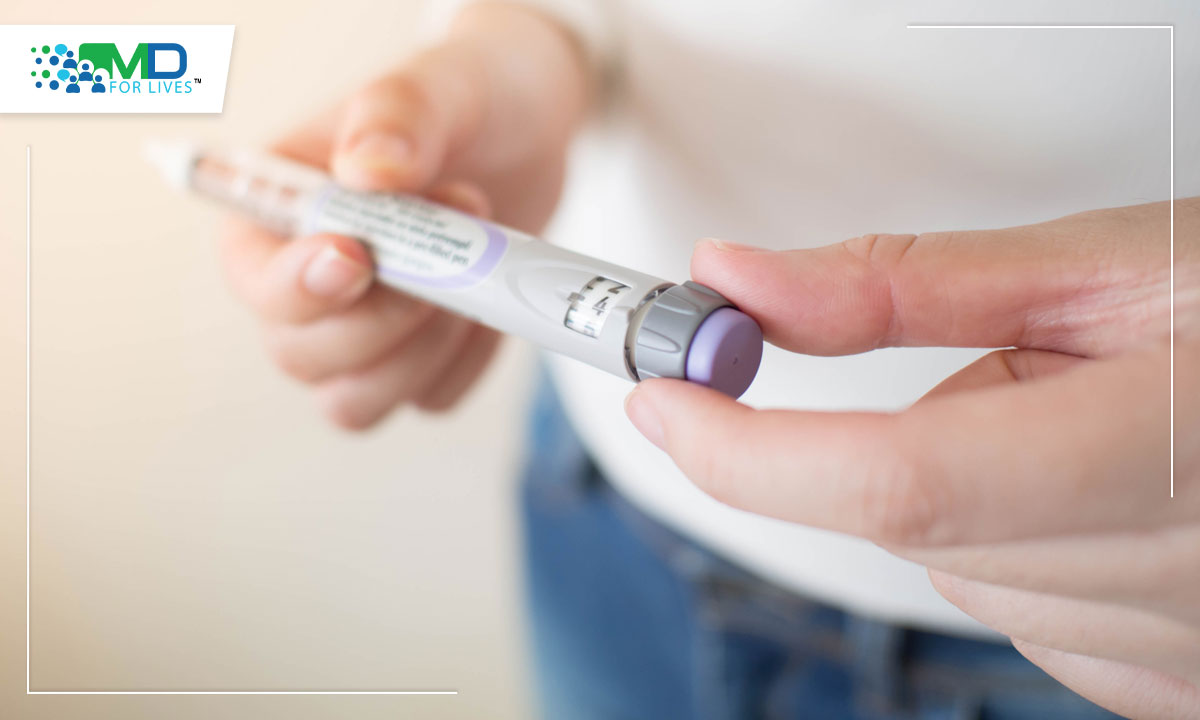
In May, the US FDA approved Mounjaro (tirzepatide) for adults with Type 2 diabetes who are also using diet and exercise to manage their disease. Tirzepatide is the first drug that targets both glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) receptors, which help regulate insulin release in the body.
Multiple diabetes drugs that target GLP-1 already exist – semaglutide is one example. In trials, tirzepatide showed effectiveness at reducing blood sugar and performed better than semaglutide or long-acting insulins. Tirzepatide is also under investigation in the treatment of obesity.
Type 2 diabetes mellitus
Type 2 diabetes mellitus (T2DM) is a chronic disease in which the body’s cells become resistant to insulin and/or insulin secretion is impaired, which leads to hyperglycemia. T2DM is mostly diagnosed in adulthood but rates are increasing among children and adolescents. It is estimated that more than 30 million Americans have T2DM.
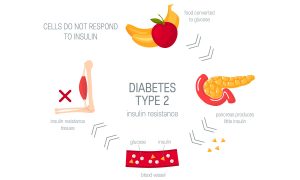
Image caption: The pancreas releases less insulin; these actions increase blood glucose levels.
Many new glucose-lowering medications have become available in the last 15 years, including GLP-1 receptor agonists (e.g., exenatide and semaglutide), dipeptidyl peptidase 4 (DPP4) inhibitors (e.g., sitagliptin), and sodium-glucose co-transporter 2 (SGLT2) inhibitors (e.g., canagliflozin). However, many patients still do not reach their target glucose levels.
Like GLP-1, GIP is another incretin gut hormone that regulates blood glucose levels through its actions on insulin.
Lifestyle changes and weight management are important parts of diabetes management, so having diabetes medications that also control body weight or help reduce weight would be advantageous for patients with diabetes.
Tirzepatide FDA approval for treatment of diabetes
The FDA approval of tirzepatide is based on the results of the five SURPASS clinical trials. Four of these trials investigated the efficacy and safety of tirzepatide as a monotherapy; tirzepatide was compared to placebo (SURPASS-1), compared with the injectable GLP-1 receptor agonist semaglutide 1 mg (SURPASS-2), or compared to the long-acting insulin analogs insulin degludec (SURPASS-3) and insulin glargine (SURPASS-4). SURPASS-5 tested tirzepatide plus insulin glargine against insulin glargine alone.

Image caption: Tirzepatide is both a GLP-1 and GIP receptor agonist that is now approved by the FDA for the treatment of diabetes.
When the maximum recommended dose of tirzepatide (15 mg) was used, the HbA1c-lowering effect was better than that of other antidiabetic medications (0.5% greater vs semaglutide, 0.9% greater vs insulin degludec, and 1.0% greater vs insulin glargine).
The SURPASS clinical trials showed that tirzepatide also had a weight loss effect in patients with diabetes. Patients who received 15 mg tirzepatide had a weight loss of more than 12 lbs compared with semaglutide, which is also a GLP-1 weight loss medication. Compared with patients on long-acting insulins (who often gained weight), patients in the tirzepatide group lost 27–29 more pounds from their initial weight.
Tirzepatide for the treatment of obesity
The clinical trial SURMOUNT-1 investigated tirzepatide as a GLP-1/GIP weight loss medication in patients who do not have diabetes. In this trial, tirzepatide significantly reduced and sustained weight loss in obese patients. Ninety-one percent of patients who received the maximum dose of 15 mg of tirzepatide had 5% or more weight reduction compared with 35% of patients who received placebo. In addition, 57% of patients in the 15-mg group vs 3% in the placebo group had a 20% or more weight reduction.
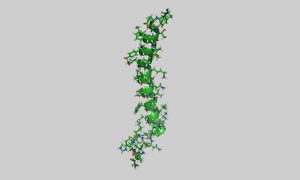
Tirzepatide is partially based on the structure of the glucose-dependent insulinotropic polypeptide (GIP) peptide hormone (pictured) but has been modified to bind to both GIP and GLP-1 receptors. (Public domain image by Ayacop, from https://en.wikipedia.org/wiki/Gastric_inhibitory_polypeptide#/media/File:2OBU.png )
Tirzepatide side effects
Side effects associated with tirzepatide were similar to those with other incretin hormone medications, which include nausea, vomiting, diarrhea, decreased appetite, upper abdominal discomfort, constipation, and abdominal pain. Compared to semaglutide, more patients in SURPASS-2 treated with tirzepatide had hypoglycemia (1.7% with 15 mg tirzepatide vs 0.4% with 1 mg semaglutide).
Tirzepatide cost
After the approval, according to Medscape Medical News, Lilly (which is marketing tirzepatide) set the drug’s price at about $12,666 per year, which is close to the price of semaglutide.
The FDA approval of tirzepatide as an antidiabetic agent and its promising antiobesity effect seen in clinical trials provide patients with diabetes an additional option to manage their diabetes and obesity.

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.