The US Food and Drug Administration has now permitted sale of a device that uses a small balloon to treat Eustachian tube dysfunction (ETD). Nearly 35 males and 28 females per 100,000 population under the age of 15 years visited an emergency department with otitis media and eustachian tube disorders in the US 2001. [1] A UK- based nationwide study on hearing found that 0.9% of 2708 adults were agreed to have a Eustachian tube dysfunction, based on audiological assessment and otoscopic examination. Comprehensive statistics are not available for lack of a consensus on the definition of what constitutes a Eustachian tube dysfunction leading to underreporting. [2] All these people are likely to benefit from this newly approved procedure.
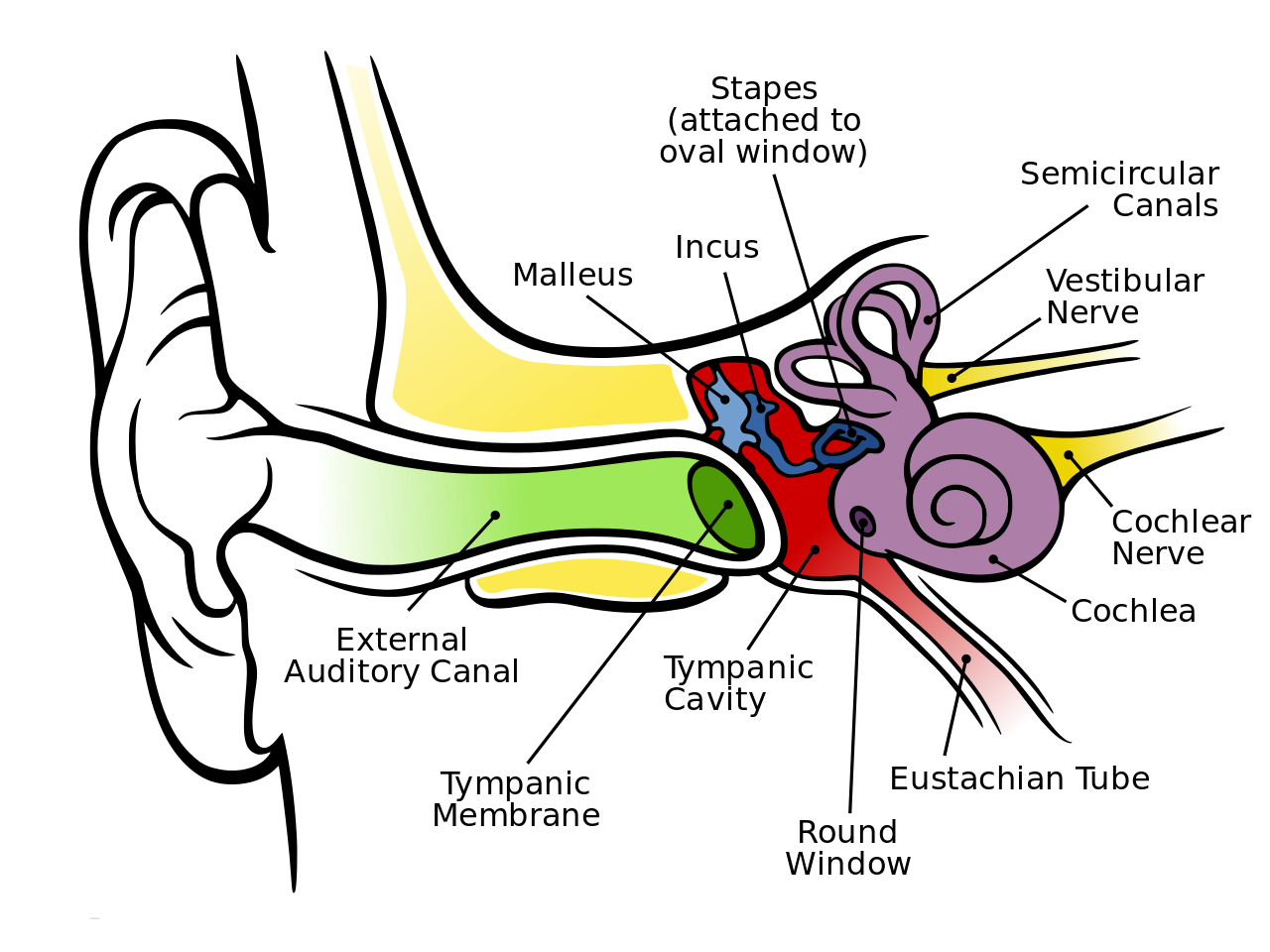
Eustachian tube dysfunction:
Laryngopharyngeal reflux into the Eustachian tube lumen results in mucosal edema, inflammation, narrowing and dysfunction which results in discomfort to the person, impairment in hearing, persistent infections in the ear, ringing in ears etc. Restoring the function to the middle ear, which is necessary to maintain equilibrium, may provide relief from the pain, any discomfort and the sensations of ear fullness or blockage associated with Eustachian tube dysfunction. [3]
Balloon for treatment of Eustachian tube dysfunction:
The development of minimally invasive procedures such as balloon dilatation of Eustachian tube (BDET) is an alternative to the Grommet tympanum membrane. The BDET is applied after the elimination of factors which influence the Eustachian tube and middle ear functioning. The BDET was offered to four patients (3 men and 1 woman) after subjective, physical, otorhinolaryngological and audiometric examinations including audiometry, pressure swallow test and tympanometry. Inclusion criteria were adults with a diagnosis of Eustachian tube dysfunction based on symptoms and whose tympanograms were abnormal. Patients with abnormal findings in craniofacial parts or active infection were excluded from study. [4]

With BDET, a catheter with a small balloon is inserted through the patient’s pharyngeal opening into the Eustachian tube. Once inflated, the balloon opens up the pathway for both mucus and air and helps to restore function to the eustachian tube. After the eustachian tube deflates, the balloon is removed.
As the method was novel, pre interventional CT angiography of the carotid arteries was performed in every patient. Complications arising during and after the procedure (bleeding or damage of regional mucosa) were noted. The clinical study assessed the feasibility and safety of the BDET during a short-term period- a 6-week observation. The patients revealed significant improvement in reducing the air-bone gap. On performing the Valsalva maneuver conducted after a week, and again after 6 weeks, three patients reported hearing improvement though the changes were reported at a different time and to a different degree. [4]
Patients under 22, who have a carotid artery that protrudes through the gap in the bone surrounding the Eustachian tube or whose Eustachian tube is always open, are contra-indicated from opting for this treatment as there can be scarring, rarely ear infections, pain, permanent hearing loss and there is a risk of rupture of a major artery (carotid). [4]
The BDET is an effective surgical intervention for the treatment of eustachian tube dysfunction. However, further studies will be necessary to determine long-term effectiveness.
Credit: Dr. Rachita on behalf of Borderless Access
Copyright © 2016 BorderlessAccess
References:
- Statistical Abstract of the United States: 2003
- Browning GG, Gatehouse S. The prevalence of middle ear disease in the adult British population. Clin Otolaryngol Allied Sci 1992;17:317–21.
- Poe D, and Gopen Q. Eustachian Tube dysfunction. In: John Jacob Ballenger, James Byron Snow [eds]. Ballenger’s Otorhinolaryngology: Head and Neck Surgery, Volume 1. Shelton, Connecticut: People’s medical publishing house;2009.
- Jurkiewicz, D., Bień, D., Szczygielski, K. et al. Eur Arch Otorhinolaryngol (2013) 270: 1157. doi:10.1007/s00405-012-2243-9.





2 Comments
Hearing Loss: How dangerous headphones for your hearing?
5 years ago[…] means that one in every five teenagers has their ears impaired due to excessive volume and headphone usage. Listening with headphones has gone quite extensive, […]
Do earphones give hearing loss? - MDForLives
4 years ago[…] means that one in every five teenagers has their ears impaired due to excessive volume and headphone usage. Listening with headphones has gone quite extensive, […]