The U.S Food and Drug Administration (FDA) has approved the first clinical trial of CRISPR to correct sickle cell disease mutation.
Sickle cell disease (SCD) is the most common hereditary disease, affecting approximately 100,000 Americans, the majority is African-American births (estimated to be 1 in 365 African descent).1 Mutation in the beta-globin gene found on chromosome 11 is responsible for sickle cell disease. Sickle cell causes chronic anemia, organ damage, and early mortality. Current treatments include medications and blood transfusions. Hematopoietic stem cell transplantation is the only curative therapy available.
Researchers in Innovative Genome Institute (IGI) – a joint effort by the University of California, Berkeley and UC San Francisco- sought to repair the mutations that cause sickle cell disease.2 After 6 years of research, FDA has now approved CRISPR genome editing technology to test in humans, to correct mutations in the hemoglobin-beta gene.
Sickle Cell Disease
Sickle cell disease was first described in Western literature in 1910 when Dr. James Herrick mentioned the strangely shaped red blood cells in a case of severe malaise and anemia. More than 100 years later, it was discovered that single base substitution in beta-globin is the cause of the changes in the red blood cells.3
Sickle cell trait (HbAS) is known to protect against Plasmodium falciparum malaria, and the majority of disease burden is in the regions where malaria is endemic including sub-Saharan Africa, India, Middle East, etc.
The different forms of sickle cell disease include Hb SC, Hb SS, Hb S beta-thalassemia, etc. Sickle cell trait is caused by abnormal hemoglobin HbS resulting from point mutation, in which A is replaced with T in codon 6 of the beta-globin chain. The mutation leads to the replacement of glutamic acid with valine at position 6 of the beta-globin chain which in turn changes red cells into a sickle.4
Hemoglobin S has reduced oxygen-binding capacity and the sticky, sickle-shaped blood cells can cause occlusion of blood vessels leading to cerebral ischemia.
CRISPR-Cas9 Gene Editing for Sickle Cell Disease
In a recent study published in the New England Journal of Medicine,5 the researchers used CRISPR-Cas9 technology to increase fetal hemoglobin levels. The fetal hemoglobin gene is usually suppressed in children and adults. Researchers reactivated the fetal gene instead of fixing the defective gene. The fetal hemoglobin can replace the defective hemoglobin.6 The treatment alleviated symptoms in at least three patients.2
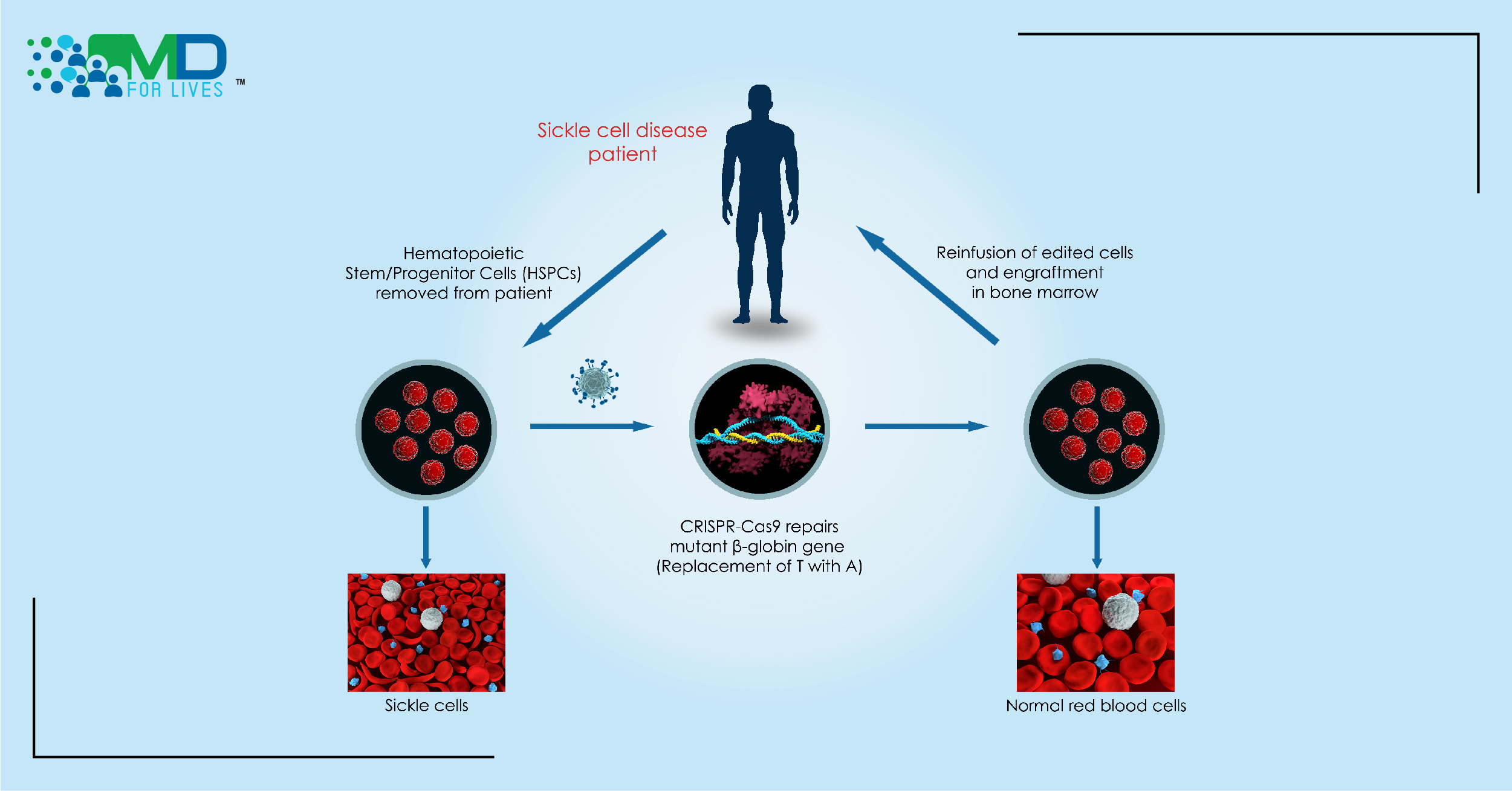
In the new trial, researchers use CRISPR gene-editing technology to correct the mutation by replacing the defective-beta globin gene with the correct copy of the gene.
The researchers developed CRISPR_SCD001, a patient-specific hematopoietic stem cell therapy in which the CRISPR-Cas9 nuclease is used- Cas9 protein and guide RNA (gRNA) targets the defective region of the beta-globin gene along with a DNA segment with customized sequence required to correct the defective gene- to stimulate repair of sickle cell disease mutation.2
In this technique, the patient’s blood stem cells undergo electroporation in which the electrical pulses increase the permeability of the cell membrane by causing pores in the membrane. The CRISPR-Cas9 enters the blood stem cells through the pores and reaches the nuclei to correct the mutation. The gRNA guides the Cas9 protein to the specific spot, the Cas9 binds to the target sequence and induces the double-strand break, and following the DNA cleavage, the break is repaired. The gene-edited stem cells are then injected back into the patient’s bloodstream.7
The goal of this gene-editing technology is to fix the sickle cell disease-causing mutation in adequate stem cells so that the resulting blood circulation will have enough gene-corrected red blood cells. The electroporation with CRISPR, a virus-free genome editing method was validated in preclinical safety/toxicology evaluation studies which were performed after consultation with FDA.7
Future of CRISPR Gene Editing
Currently, the CRISPR protocols are ex vivo, the cells are taken out of the body, correct the mutations responsible for the disease, and transplanted back. Right before the infusion of stem cells, the patients are subjected to high-dose chemotherapy to kill the existing stem cells in the bone marrow that clear the way for transplanted cells to flourish.2
Researchers from IGI are working to improve the technique, to correct the sickle cell disease mutation in the hematopoietic stem cells inside the body without removing them or destroying the bone marrow. Removing the bone marrow can weaken the immune system and increase the risk of infection or cancer.
In gene therapy and gene editing, the stem cells are extracted from the patient so it is safer than transplantation from another person.7 The cure for sickle cell disease can be accessible and affordable to patients globally and the FDA approval for this trial is the first step on that path.