When HIV develops resistance to commonly used drugs, patients and doctors are faced with a difficult situation. Some patients have multidrug-resistant HIV therapy and have few options left.
A potential antiretroviral drug called lenacapavir uses a new mechanism that is not employed by any current HIV drug. Lenacapavir targets the HIV viral capsid, making it active against multiple stages of the viral life cycle, and is long-acting. These features increase the chances the new drug will be effective against multidrug-resistant HIV.
A recent lenacapavir Phase 3 clinical trial with 72 HIV-positive patients reported promising results. Lenacapavir is also being investigated for use in HIV pre-exposure prophylaxis.
Antiretroviral Resistance:
Patients can harbor drug-resistant HIV either because the virus has developed drug-resistance mutations during the patient’s treatment or because the strain that infected the patient was already drug-resistant (primary resistance). With multidrug-resistant HIV, patients may be unable to maintain viral suppression on treatment, which puts them at risk of developing AIDS.

Lenacapavir Mechanism and History
The HIV capsid is a shell, consisting of protein subunits, that surrounds the viral genome and its associated proteins. To infect a host cell, the virus must “uncoat,” or disassemble its capsid, to release the viral nucleic acid. Later, capsid subunits must assemble around newly replicated viral RNA before the virus is released from the host cell. Lenacapavir is a first-in-class potential HIV therapy that interferes with both early and late capsid functions, thus attacking the virus at two points in its life cycle.
In February 2022, the FDA sent a Complete Response Letter to Gilead, the company developing lenacapavir, because of concerns regarding compatibility between the drug and the borosilicate glass vials used to contain it. A clinical hold was placed on lenacapavir trials. The clinical hold has since been lifted, and on June 27, 2022, Gilead resubmitted its lenacapavir New Drug Application (NDA) for treatment of multi-drug resistant HIV-1 infection, along with evidence that a different kind of glass vial can be used safely.
The CAPELLA Trial
Lenacapavir was studied in the Phase 3 CAPELLA trial, which was a 52-week, international study conducted in 11 countries. All patients were adults or adolescents (ages 12 and up) who had multi-drug resistant HIV-1 infection, with their current HIV therapy failing to control the infection. Most of the patients had severe immunodeficiency, and patients had tried a median of nine antiretroviral drugs.
The study included a placebo-controlled cohort (cohort 1) of 36 patients, who were randomized at a 2:1 ratio to receive either oral lencapavir or placebo, along with their existing ineffective therapy, over 15 days. After the initial 15-day period, patients in the lenacapavir group received lenacapavir injections every six months (maintenance period), while those in the placebo group switched to lenacapavir, with oral dosing over 15 days followed by injections every six months.
The second cohort included 36 patients who did not qualify for or were not evaluated in time for the first cohort. These patients participated in an open-label study with an initial period of oral lenacapavir followed by injections of the drug every six months.
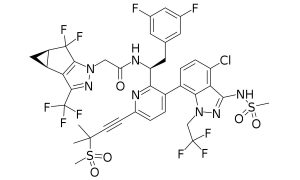
Structure of lenacapavir (Public Domain image by Reba16, from https://commons.wikimedia.org/wiki/File:Lenacapavir.svg)
During the first 15 days in cohort 1, 88% of active treatment and 17% of placebo patients achieved a reduction in the RNA viral load from baseline of at least 0.5 log10 copies per milliliter plasma, which was the primary endpoint. At week 26, while on lenacapavir, 81% of Cohort 1 patients and 83% of Cohort 2 patients had a viral load below 50 copies per milliliter. Patients’ CD4+ counts increased during lenacapavir dosing by a least-squares mean of 75 in cohort 1 and 104 in cohort 2.
Nineteen CAPELLA patients were evaluated for the emergence of resistance to lenacapavir. Eight had viral resistance mutations emerge, and four of these had resuppression of the virus.
No serious adverse events were deemed to be related to the study drug. One cohort 2 patient with severe immunodeficiency and a history of non-Hodgkin’s lymphoma died from cancer during the study. The most common adverse events were injection-site reactions, nausea, diarrhea, and constipation.
Lenacapavir is under investigation as pre-exposure prophylaxis
Lenacapavir is long-acting and can be administered by injection at intervals of up to 6 months, which may make it useful as a pre-exposure prophylaxis option.6 Because of the drug’s long duration of effects, it will be important to investigate the drug’s safety during pregnancy and breastfeeding.2 Lenacapavir’s efficacy at preventing HIV infection is being investigated in several studies.

References:
- https://www.nejm.org/doi/full/10.1056/NEJMoa2115542?query=TOC&cid=NEJM%20eToc,%20May%2012,%202022%20DM1031907_NEJM_Non_Subscriber&bid=971783109
- https://www.nejm.org/doi/full/10.1056/NEJMe2204376?query=recirc_curatedRelated_article
- https://clinicalinfo.hiv.gov/en/drugs/lenacapavir
- https://www.contagionlive.com/view/fda-lifts-clinical-hold-of-lenacapavir-for-hiv-treatment-and-prevention
- https://www.formularywatch.com/view/gilead-resubmits-nda-of-long-acting-lenacapavir-for-hiv
- https://www.poz.com/article/lenacapavir-shows-promise-longacting-hiv-treatment-prevention

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.






