FDA has authorized marketing for the first-of-its-kind device to assist upper limb rehabilitation of stroke patients.1
In 2017, a study published in the journal Stroke demonstrated the therapeutic potential of IpsiHand exoskeleton driven by the brain-computer interface (BCI) in stroke survivors.2
The IpsiHand Upper Extremity Rehabilitation System, developed by Neurolutions Inc. licensed from the Washington University School of Medicine in St. Louis has now received marketing authorization from FDA.
Motor impairments after stroke
Stroke is one of the leading causes of mortality and long-term disability in adults. Data suggest nearly 15 million have a stroke every year, the annual mortality is approximately 5 million, and 5 million survivors are chronically disabled.3 The prevalence and incidence rate varies among populations. It is estimated that more than 795,000 people in the United States suffer a stroke every year.4 Stroke has a huge public health burden and is expected to increase in the subsequent decades largely due to demographic changes, particularly in developing countries.5
The prevalence of motor function deficit after stroke is significantly high. The degree of disability that follows a stroke varies from mild to severe, nearly 88% of the patients with stroke have hemiparesis, affecting lower and upper limbs.6 The motor impairment caused by a stroke can affect patient’s mobility, limit their functional activities of daily living (ADL), and ultimately affect their quality of life. The main goal of rehabilitation technologies is to recover motor function and maximize functional independence.
Upper limb impairments after stroke
Most of the daily activities involve upper limbs. The upper limb dysfunction can profoundly increase the dependence in stroke patients because the participation in completing daily basic functional tasks that involve reaching, grasping, and manipulating objects is primarily affected in stroke patients. During initial rehabilitation, many stroke patients recover some walking function but the majority of the patients with upper limb impairment show less improvement and still would not be able to incorporate the upper extremity in their daily activities. It is found that following initial therapy, nearly 65% of stroke patients show nonfunctional upper extremity and 30% showed partial recovery but still fail to perform daily activities.7
Several tests are available to evaluate the upper limb motor functions for appropriate therapeutic interventions. Lately, several new therapies have emerged to restore motor functions but still there remains a need for the treatment-oriented device and assisted device to increase the functional performance of the upper limb.
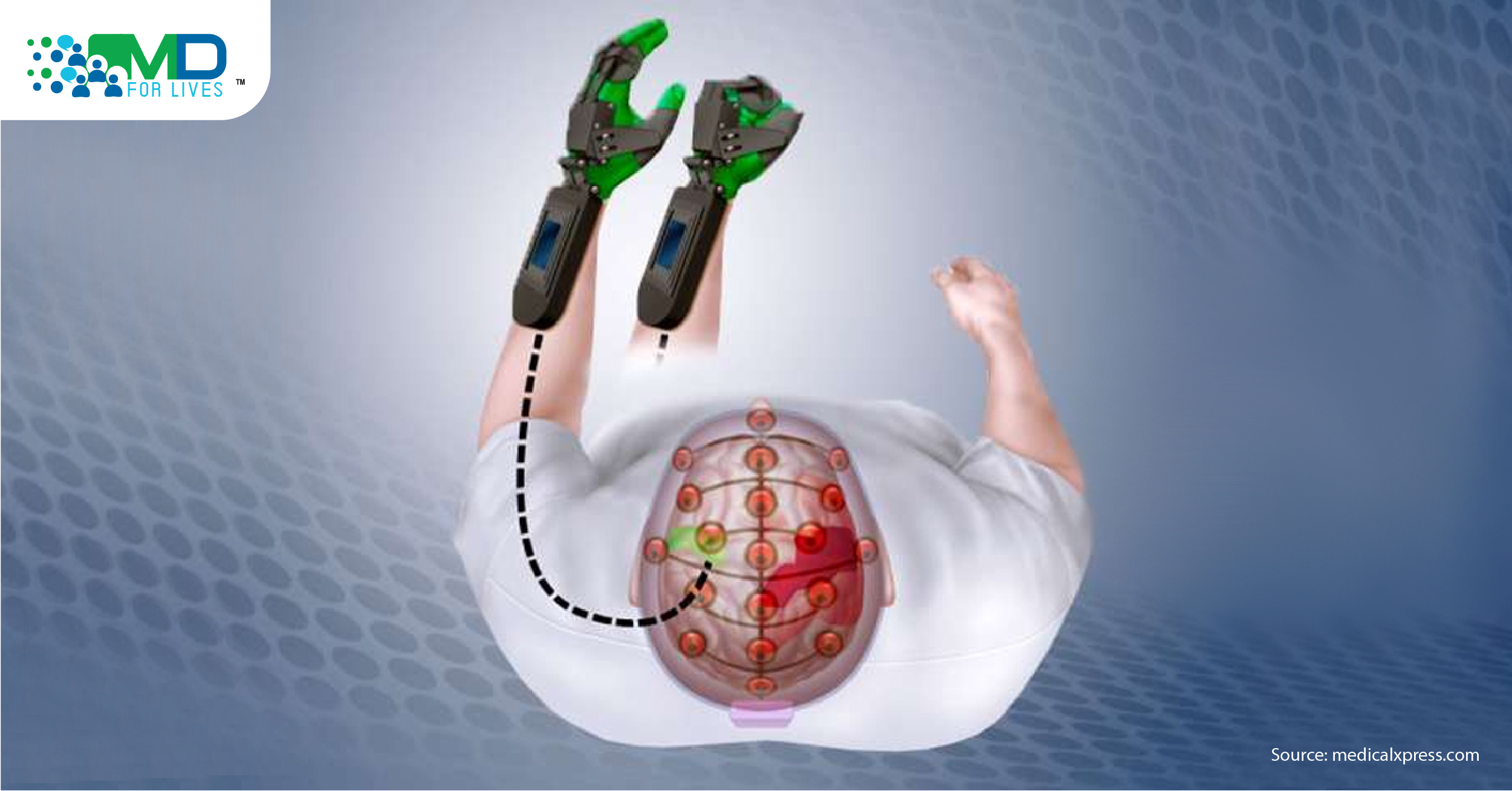
IpsiHand, the first BCI-controlled stroke-rehabilitation device
Behavioral therapies such as constraint-induced movement therapy, robot-aided sensorimotor stimulation, etc., can improve motor function in the upper extremities. However, a certain level of peripheral motor function is vital to engage with such behavioral therapies2 and that presses the need for an alternative approach that can directly engage with the central nervous system. Researchers found that the brain-computer interface (BCI) detects electrical signals from the brain and sends feedback to the brain and induces plasticity and thus restores motor function in stroke patients.2
The left hemisphere of the brain controls movement of the right side of the body and vise versa. Right hemisphere stroke (damages the motor cortex of the right side of the brain) results in left-sided weakness. However, the left hemisphere of the brain is intact and can still generate signals. BCI-driven neurorehabilitation approach detects the signal from the uninjured left hemisphere of the brain and translates the signal to the left upper limb and thus recovers the control of the affected limb.
Researchers created an IpsiHand System device, a powered exoskeleton driven by the BCI system comprised of non-invasive electroencephalography (EEG) amplifier, electrodes, and movable braces that fit the patient’s hand.8 The device detects the patient’s thoughts to open and close the affected limb in a 3-finger pinch grip.2 The device detects signals from the unaffected hemisphere associated with the intended motor function to move the paretic limb.1
FDA evaluated the safety and effectiveness of the device using a data of 12-week clinical trial which included 40 patients.1 The motor skills of the patients were assessed before, during, and after the treatment. The ability of grasp, grip, and pinch strength was measured. After 12 weeks of using the device, all 40 patients showed improvement in motor function, their Action Research Arm Test (ARAT) score increased an average of 6.2 points.2 The findings showed the therapeutic potential of the new IpsiHand System device which uses the signals generated from the unaffected hemisphere and converts them into hand movements.
The IpsiHand device is not recommended for patients with severe upper limb spasticity or hand and finger joint contractures. Also, not recommended for patients with skull craniotomy.
FDA has granted “Breakthrough Device” status as well as the De Novo marketing authorization for Neurolutions’ IpsiHand Upper Extremity Rehabilitation System.
The device is recommended for patients (aged 18 or older) with chronic stroke undergoing stroke rehabilitation.