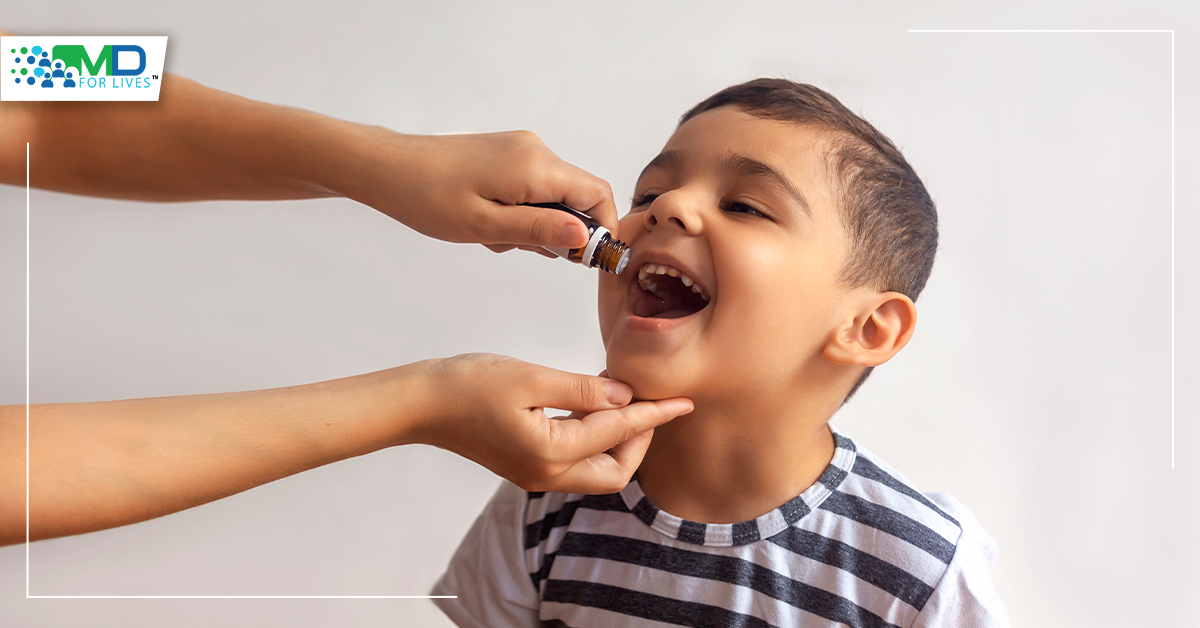
The Immune Tolerance Network IMPACT trial found that giving peanut oral immunotherapy to extremely peanut-allergic children aged 1 to 3 years successfully desensitized most of them to peanuts and resulted in remission of peanut allergy in one-fifth of them. The ground breaking findings of the IMPACT study imply that there is a window of opportunity in early childhood to induce peanut allergy remission with oral immunotherapy.

Peanut allergy
The majority of serious food-related allergies are caused by peanuts. It usually appears early in childhood, and those who are affected do not usually outgrow it. Trace amounts can cause an allergic reaction in highly sensitized people. Peanut allergy is a condition that requires special attention.
Peanuts cause a type I hypersensitivity reaction that is solely mediated by IgE. Peanut-specific IgE antibodies bind to high-affinity receptors on mast cells and basophils in such reactions. At least seven peanut proteins have been identified as causing allergic reactions. When peanut allergens pass across mucosal barriers, cell-bound IgE and peanut allergens crosslink, causing preformed allergic mediators to degranulate and cells to activate. These cells may then release a range of cytokines and chemokines, which attract other inflammatory cells and contribute to the late-phase allergic reaction mediated by IgE.
Most youngsters have their first allergic reaction to peanuts between the ages of 14 and 24, and this reaction usually occurs at home. According to a voluntary registry, around half of all children with peanut allergies exhibit allergic manifestations in one target organ system, 30% in two systems, 10% to 15% in three systems, and 1% in four systems.
About 200 mg of protein is found in a single peanut. The majority of persons with peanut allergies experience symptoms after eating less than one peanut, and extremely allergic people can react to trace amounts. Symptoms appear in more than 70% of children with a peanut allergy after their first reported exposure.
A medical history and physical examination are used to diagnose a potential food allergy. The presence of peanut-specific IgE can be validated using a skin prick test or a fluoroenzyme immunoassay. Oral food challenges might be used when there is uncertainty about the diagnosis.

Genes driving peanut allergy
Gene expression studies in food allergic people have revealed some of the molecular pathways that may underpin peanut allergy. Differentially expressed genes found in food allergic people suggest that underlying immune system malfunctions play a role in the development of food allergies.
The combined interpretation of the lme models, WGCNA, and KDA indicated six essential driver genes in particular (LTB4R, PADI4, IL1R2, PPP1R3D, KLHL2, and ECHDC3), as these were the only genes that matched all three requirements of being:
- Peanut genes with a substantial change in expression when exposed to peanuts but not when exposed to a placebo
- Members of the WGCNA peanut response module, and
- Top-level important driver genes in the hierarchical visualization of the network, anticipated to causally modify the state of peanut genes and peanut response module members at the most proximal level.
Oral immunotherapy induces desensitization or remission of peanut allergies in young individuals
Dietary avoidance is the current standard of care for young children with peanut allergies. A randomized, double-blind, placebo-controlled study was carried out in five US academic medical centers to assess if peanut oral immunotherapy may lead to desensitization (an elevated allergic reaction threshold while on therapy) or remission (a condition of non-responsiveness after stopping immunotherapy). Children aged 12 to 48 months who were reactive to 500 mg or less of peanut protein during a double-blind, placebo-controlled feeding challenge were eligible.
Participants were randomly randomized to receive peanut oral immunotherapy or placebo for 134 weeks (2000 mg peanut protein per day), followed by 26 weeks of avoidance, using a computer, in a 2:1 allocation ratio, with participants, study staff, and investigators masked to group treatment assignment. The primary result was desensitization at the conclusion of therapy (week 134), and the key secondary endpoint was remission following avoidance (week 160) in the intention-to-treat population, which was measured by DBPCFC to 5000 mg. In the same population, safety and immunological characteristics were examined.
Between August 13, 2013, and October 1, 2015, 146 children with a median age of 393 months were randomly assigned to receive either peanut oral immunotherapy (96 participants) or a placebo (50 participants). At week 134, 68 of 96 subjects who received peanut oral immunotherapy met the primary endpoint of desensitization, compared to one of 50 who received placebo.
During week 134 of the DBPCFC, the median cumulative tolerated dose for peanut oral immunotherapy was 5005 mg versus 5 mg for the placebo. After avoiding peanuts, 20 of the 96 patients who received peanut oral immunotherapy reached the remission criterion, compared to one of the 50 who received the placebo. Peanut oral immunotherapy had a median cumulative tolerable dose of 755 mg during week 160 DBPCFC, while the placebo had a dose of 0 mg.
Younger age and lower baseline peanut-specific IgE were predictive of remission in subjects receiving peanut oral immunotherapy, according to multivariable regression analysis. The majority of individuals (98% with peanut oral immunotherapy vs. 80% with the placebo) experienced at least one oral immunotherapy dose reaction, which was mostly mild to moderate and occurred more frequently in the peanut oral immunotherapy group. In 21 subjects undergoing peanut oral immunotherapy, 35 oral immunotherapy dosage events with mild symptoms were treated with epinephrine.
Peanut oral immunotherapy started before the age of 4 years was linked to an increase in both desensitization and remission in children with a peanut allergy. Immunological indicators were linked to the onset of remission. The findings point to a window of opportunity for intervention at a young age to promote peanut allergy remission.

https://www.ncbi.nlm.nih.gov/pmc/articles/PMC154188/
https://www.nature.com/articles/s41467-017-02188-7
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)02390-4/fulltext