In a recent study, researchers have offered a detailed analysis of the most innovative advancements in basic and applied cancer research. Cancer is the world’s most significant cause of death, with approximately ten million fatalities expected by 2020.
Even though medicine has progressed significantly, several concerns must be addressed to improve cancer treatment. As a result, oncological research is focusing on developing novel, practical therapies that can mitigate the harmful effects of current treatments. A few of them are given below:
Nanomedicine:
Biocompatible nanoparticles are remote systems (1–1,000 nm in size) with unique physicochemical features due to their small size and high surface-to-volume ratio. They are utilized in cancer treatment to address challenges such as limited specificity and bioavailability of medicines and contrast agents.
As a result, encapsulating active drugs in nanoparticles will improve their solubility/biocompatibility, stability in body fluids, and tumor vasculature retention time. Nanoparticles can also be highly selective for a specific target and release drugs in a controlled manner by responding to a particular stimulus.
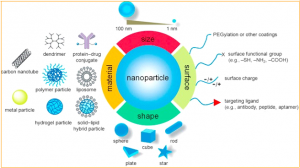
Figure depicting NPs (nanoparticles) classified based on their composition, size, surface, and shape.
Use of Extracellular vesicles for cancer diagnosis and therapy
Based on their biogenesis, EVs are divided into two groups. Exosomes, which are small vesicles with a size of 30–150 nm that originate from endosomes in physiological and pathological conditions, and shed microvesicles (sMVs), which have a size of 50–1,300 nm and can be found in almost any extracellular bodily fluid.
Nowadays, one of the most pressing concerns in cancer diagnosis is the early detection of biomarkers using non-invasive approaches. In recent years, exosome identification has been confirmed as a trustworthy method for preclinical practice in several cancer treatments. Exosomes could also be used to deliver drugs to cancer patients as natural, biocompatible, and low immunogenic nanocarriers. New ways for producing ad hoc exosomes have recently been devised. Exosome-producing cells have been genetically manipulated to overexpress specific macromolecules or to release exosomes containing specific targeting molecules.
Many challenges surrounding exosome clinical translation remain unresolved, the majority related to the development of preclinical methodologies for exosome separation, quantification, storage, and standard drug loading protocols.
Natural Antioxidants in Cancer Therapy
Exogenous insults, such as UV radiation, air pollution, and tobacco smoke cause the creation of reactive species, particularly oxidants and free radicals, which are responsible for cancer development. These molecules can be formed by medicine delivery to the individual, but they can also be produced spontaneously within our bodies via normal physiological aerobic processes.
Our bodies’ preventive capabilities against these chemicals are sometimes insufficient to counteract the massive damage they cause. Therefore, natural antioxidants such as vitamins, polyphenols, and plant-derived bioactive substances have recently been studied in the hopes of introducing them as preventive agents and potential therapeutic medications. These compounds have been screened in vitro and tested in vivo, demonstrating significant anti-proliferative and pro-apoptotic effects, and have been introduced as supplementary cancer therapeutics.
Despite the benefits of natural medications, their practical implementation is challenging due to their low bioavailability and toxicity.
Targeted Therapy and Immunotherapy
One of the significant drawbacks of traditional cancer treatment is that most medications affect both healthy and cancerous tissues, resulting in serious side effects. So researchers are working hard to figure out how to target only the desired site.
There are two ways to accomplish targeted therapy. The first is passive targeting, which relies on the small size of nanoparticles to assault neoplastic tissues’ leaky vasculature and poor lymphatic outflow. Whereas the other is active targeting, which improves tumor cell uptake by targeting specific receptors that are overexpressed on them. On the other hand, passive targeting is challenging to regulate and can result in multidrug resistance (MDR).
Adoptive cell transfer (ACT) is a method of immunotherapy that involves collecting T-lymphocytes (T-cells) with the most potent anti-cancer activity straight from the patient’s blood, growing them ex vivo, and then reinfusing them back into the patient. Autologous T-cells can be genetically modified in-vitro to express a chimeric antigen receptor (CAR), making them more selective towards antigens found on cancer cells.
Despite these encouraging findings, substantial work is still being done to understand the long-term consequences of CAR T-cell therapy and their fate within tumors and improve CAR T-cell expansion methods.
Radiomics and Pathomics: most recent advances in cancer treatment.
Radiomics and pathomics are two cutting-edge fields that use quantitative picture features from radiology and pathology screens as therapeutic and prognostic predictors of disease outcomes. Radiomics refers to the high-throughput quantification of tumor attributes derived from medical image processing. Pathomics, on the other hand, is based on creating and analyzing high-resolution tissue pictures.
To detect disease phenotypes, flexible databases are necessary to accommodate large amounts of data from gene expression, histology, 3D tissue reconstruction (MRI), and metabolic characteristics (positron emission tomography, PET). There is an immediate need to create explicit data acquisition guidelines.
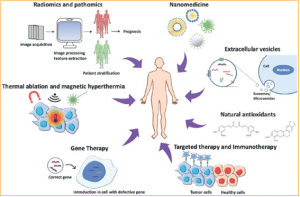
Cancer therapy approaches: The image represents the most innovative strategies to treat cancer.
What is the future of Cancer Treatment?
This paper has suggested that In recent years, cancer research has made significant progress toward more effective, precise, and minimally invasive cancer treatments in recent years. While nanomedicine combined with targeted therapy improved the biodistribution of new or previously tested chemotherapeutic agents around the specific tissue to be treated, other strategies such as gene therapy, immunotherapy, and antioxidant molecules provide cancer patients with new options. Thermal ablation and magnetic hyperthermia, on the other hand, are potential alternatives to tumor resection. Finally, radiomics and pathomics techniques aid in handling large data sets generated by cancer patients to enhance prognosis and outcomes.
Despite all of the existing limitations, with all of the advancements in cancer diagnosis and therapy in the recent decade, the future of cancer treatment appears bright.
References:
- https://www.who.int/news-room/fact-sheets/detail/cancer
- https://pubmed.ncbi.nlm.nih.gov/29115304/
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6753017/

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.