Treatment:
- Surgery is seen as the only curative option for localized colon cancer (stage 1-3), and surgical resection may be the only curative choice for stage 4 disease.
- Adjuvant chemotherapy is a standard of care for stage 3, though can also be used in stage 2 (specifically in patients with high-risk factors to predict recurrence).
- Radiation therapy is utilized in palliative therapy, specifically for bone or brain metastases.
- In stage 4 disease, chemotherapy is the popular choice, especially when weighing up increasing risk factors, like age, comorbidities, ECOG score, TNM burden, or prior exhaustive therapies.
Surgery:
Removal of the primary tumor with adequate margins of lymphatic drainage is the key to surgical resection. You can see a breakdown of standard colectomies for adenocarcinoma of the colon:
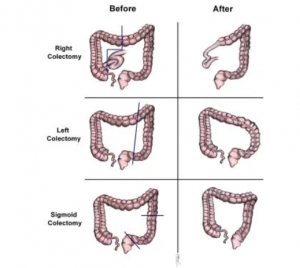
- In the case of lesions in the cecum or right colon, a hemicolectomy is recommended.
- In the case of lesions in the proximal or middle transverse colon, an extended right hemicolectomy may be performed.
- In the case of lesions in the splenic flexure and left colon, a left hemicolectomy is recommended.
- If there are sigmoid colon lesions, a sigmoid colectomy is used.
- In cases of HNPCC/Lynch syndrome, attenuated familial adenomatous polyposis (FAP), or metachronous cancers in separate colon segments, a total abdominal colectomy with ileorectal anastomosis is recommended.
- Laparoscopic surgery. There is minimal difference between open surgery and the less invasive laparoscopic approach, making the latter a preferred option among patients. The Clinical Outcomes of Surgical Therapy Study Group trial showed similar efficacies in terms of 5-year disease-free survival (69% laparoscopic-assisted colectomy vs 68% open colectomy; and 76% overall survival with LAC vs 75% open surgery).
- In Stage 4 colon cancer, a study by Venderbosch et al. argued that less than 10% of patients with asymptomatic, surgically incurable colorectal cancer required surgery for obstruction or perforation.
- A study by Brouquet et al. advocated the benefits of resection after 2nd line chemotherapy, with improvements in overall survival, progression-free survival, with acceptable safety.
- For patients with obstruction, colonic stents may be used as an alternative treatment for those patients worrying about tumor spread caused by perforation remains.
- Thermal ablation techniques, including cryotherapy (the use of probes to freeze tumors and surrounding hepatic parenchyma), could be an option for patients who cannot tolerate surgery, but risks from laparotomy, liver cracking, thrombocytopenia, and disseminated intravascular coagulation (DIC) are concerning.
- Radiofrequency ablation (RFA) (probes that heat liver tumors and surrounding margins) has minimal morbidity, though local recurrence can occur, based on tumor size.
Chemotherapy:
- The QUASAR trial showed a 3.6% benefit in 5-year survival by using adjuvant chemotherapy in stage 2 colon cancer, though ASCO recommends against it, especially in low-risk groups. Risk is based on: less than 12 lymph nodes in the surgical specimen, perineural/lymphovascular invasion, poorly differentiated/undifferentiated tumor grade, intestinal obstruction, tumor perforation, and grade bd3 tumor budding (>10buds)
- The Adjuvant Colon Cancer Endpoints group showed the advantage of adjuvant chemotherapy, on disease-free survival and reduction in recurrence for stage 2/3 colon cancer. In older patients, van Erning et al. showed that adjuvant chemotherapy reduced the risk of distant recurrence by about 50%.
- Standard chemotherapy includes 5-FU combined with levamisole and leucovorin, with several studies demonstrating a 30% reduction in 5-year recurrence risk.
- The oral fluoropyrimidine drug capecitabine is another good chemotherapy option. The -ACT study demonstrated a non-inferiority vs 5-FU/leucovorin, and efficacy benefits for older patients.
- Oxaliplatin + capecitabine (XELOX) showed a 73% overall survival vs 67% in 5-FU/leucovorin.
- The MOSAIC study showed a 5-year DFS improvement for stage 3 colon cancer when oxaliplatin was combined with 5-FU/leucovorin (FOLFOX4).
- Regarding the length of time on chemotherapy, the IDEA trial demonstrated that 3-year DFS in 3 months of FOLFOX was lower than 6 months. Though shorter therapies had less neurotoxicity. ASCO recommends 6 months of adjuvant chemo with FOLFOX for stage 3. While NCCN recommends the following: Low-risk stage III: CapeOx for 3 months or FOLFOX for 3-6 months. High-risk stage III: CapeOx for 3-6 months or FOLFOX for 6 months.
- In metastatic disease, FOLFOX 4 improved the response rate and time to progression, though was associated with an increase in neutropenia and paresthesias.
- Lonsurf (tipiracil/trifluridine) was shown to have a good OS and PFS vs placebo for patients who had previously been treated with fluoropyrimidine, oxaliplatin, irinotecan, bevacizumab, and cetuximab or panitumumab (for patients with KRAS wild-type tumors), with the following results: 7.1 months with Lonsurf vs 5.3 months with placebo, PFS 2 months with Lonsurf vs 1.7 months with placebo.
Targeted and Immunotherapies:
- In the phase III ARTIST trial, the anti-VEGF drug bevacizumab demonstrated improvements in PFS (6.2 months to 9.2 months) and OS (14.3 months to 19.3 months) when added to irinotecan, 5-FU, and leucovorin (IFL). In the CAIRO3 trial, capecitabine and bevacizumab (CAPOX-B) delayed disease progression.
- Cetuximab – an EGFR inhibitor that targets KRAS wild-type patients that can be used with irinotecan (EPIC trial), with FOLFIRI (CRYSTAL), or as monotherapy.
- Panitumumab – an EGFR inhibitor. The PRIME trial (panitumumab + FOLFOX 4 vs FOLFOX alone) indicated the benefit for patients with wild-type KRAS tumors (PFS 9.6 vs 8 months), while the ‘0007 trial showed (OS 10 months with panitumumab and best supportive care vs 6.9 months BST alone). In the PRIME trial, patients with mutant KRAS had reduced PFS.
- Ramucirumab – for patients that have progressed with first-line standard of care, the RAISE trial showed the benefit of ramucirumab–FOLFIRI vs placebo–FOLFIRI (PFS 13.3 mo vs 11.7 mo; OS 5.7 mo vs 4.5 mo).
- Nivolumab – the PD-1 inhibitor in the CheckMate 142 trial (w/wo ipilimumab), demonstrated one year OS was 73.8%.
- Pembrolizumab – the PD-1 inhibitor for MSI-H or dMMR patients (the same as Nivolumab). In the KEYNOTE-177 trial, the risk of disease progression or death was reduced by 40%, with a PFS of 16.5 months vs 8.2 with chemo.
- Regorafenib – a kinase inhibitor for patients already treated with a number of therapies (including anti-VEGF, anti-EGFR, and KRAS WT therapies).
- Ziv-aflibercept – a decoy receptor for the VEGF-A/VEGF-B/Placental growth factor (PIGF) showed benefit in the VELOUR trial when combined with FOLFIRI, with improvements in OS and PFS.
- Larotrectinib – an inhibitor of tropomyosin receptor kinases (TRKA, TRKB, TRKC) encoded by NTRK genes was approved in 2018 for patients who have failed previous therapies, have solid tumors with NTRK fusions (present in 1% of colorectal cancers), have metastatic disease, and/or are likely to experience morbidity after surgery.
- BRAF V600E mutation positive metastatic colon cancer can be treated with Dabrafenib+ trametinib + cetuximab/panitumumab or encorafenib + cetuximab or panitumumab w/wo binimetinib, based on BEACON trial data (OS 9.3 mo with triplet therapy vs 5.9 mo SOC).
- HER2+’ve disease. HER2 is expressed in 3% of colorectal cancer and in 5–14% of RAS/BRAF-WT patients. Patients treated with trastuzumab and lapatinib showed 12 of 27 patients in a proof-of-concept trial still alive at 1 year, with one patient with a complete response.
Radiation Therapy:
RT is not used in the adjuvant setting in colorectal cancer, though it is an option in palliative settings for brain or bone metastases. Stereotactic RT (e.g., CyberKnife) is an option being studied, while a study by Hendlisz et al. showed that radioembolization with yttrium-90 improved time to liver progression, for unresectable chemo-refractory patients.
Other measures:
Improvements in the following criteria are also important based on numerous studies.
- Physical activity
- Waist circumference
- Smoking
- Alcohol intake
- Diet

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.