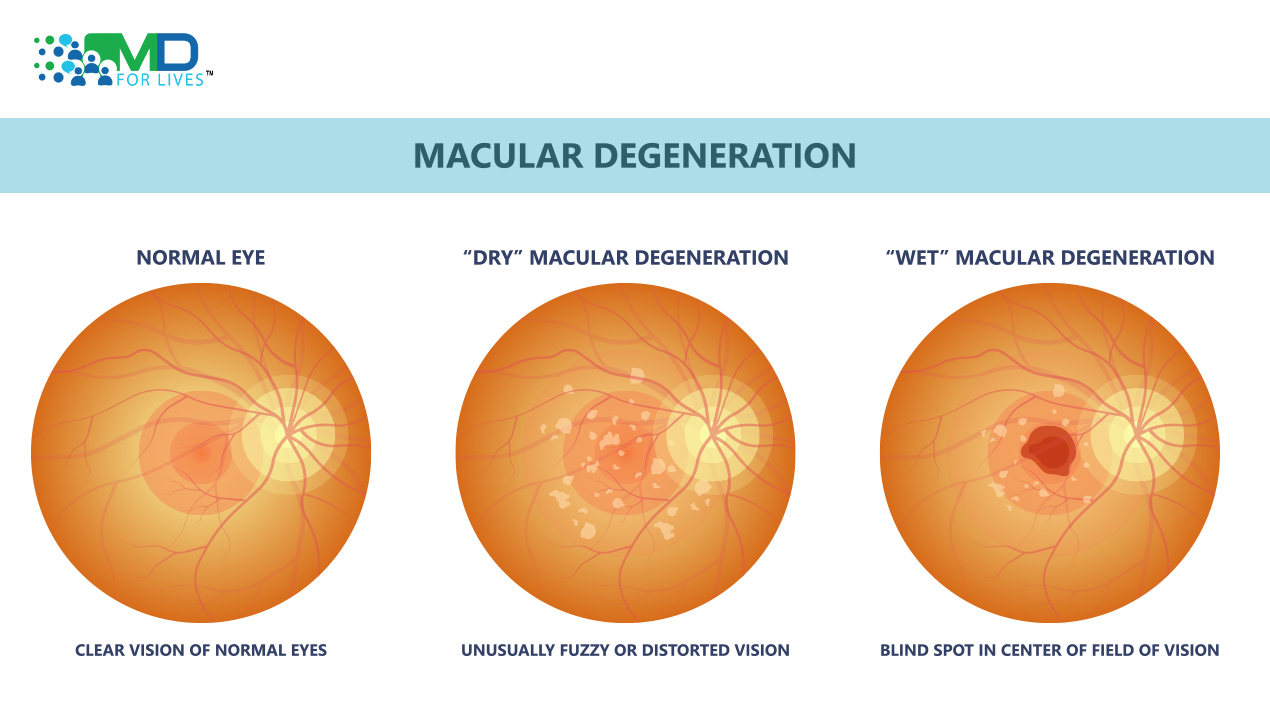
“In the United States alone, as many as 11 million people have age-related macular degeneration (AMD).”
When I was a student at Pennsylvania College of Optometry (now Salus University), there was little we could do to help prevent our patients’ vision loss from age-related macular degeneration (AMD). I did my low vision/vision rehabilitation residency at the Feinblom Vision Rehabilitation Center.
At the time, low-vision devices were one of the few options to enhance patients’ vision for those suffering from AMD. Treatments began to crop up that could stabilize visual acuity. Available treatments were limited to wet AMD. The Wet form makes up a small minority of the total cases of AMD.
“The American Macular Degeneration Foundation estimates that around 10-15% of people with AMD have the wet subtype.
The first treatment for wet macular degeneration was laser photocoagulation
The Macular Photocoagulation Study Group demonstrated that laser treatment of extrafoveal choroidal neovascular membranes lesions was beneficial in preventing or delaying large losses of visual acuity for at least 5 years.
Other treatments were developed, which included surgical removal of the choroidal neovascular membrane and photodynamic therapies. The latter involved the intravenous infusion of a photosensitive drug.
The second step involved activating the drug by nonthermal light at a specific wavelength5,6 This damage may lead to platelet activation and subsequent thrombosis and occlusion of choroidal neovasculature within the treated area.
Vascular endothelial cell growth factor was targeted with a revolutionary new approach.
Anti-VEGF biologics such as Avastin, Eylea and Lucentis allow for functional improvement and normalization of macular morphology with reductions in intra- and subretinal fluid, hyperreflective material and pigment epithelial detachments.
These agents are still in wide use today. However, these injections often involve multiple sessions spread out over months. Patients may need help with follow-up. Other patients may fail to respond appropriately to anti-VEGF therapies. New implantable anti-VEGF release systems have been recently developed. However, this is a more invasive procedure and may stimulate inflammation.
Vabysmo, recently approved, has a dual mechanism of action simultaneously targeting VEGF and angiopoietin-2. Blockade of these two main drivers of angiogenesis might be more effective in preventing expansion of the neovascular complexes and thus recurrence.
The main limitation of all the above therapies is that they only address wet macular degeneration. The majority of patients with macular degeneration have the dry type.
National Institutes of Health funded AREDS to study macular degeneration.
The original AREDS study was launched in 1996. This study showed that a dietary supplement formulation:
- 500 mg of vitamin C
- 400 international units of vitamin E
- 2 mg copper, 80 mg zinc
- 15 mg beta-carotene)
could significantly slow the progression of AMD from moderate to late disease.
Five years after the clinical trial ended, the beneficial effects of the AREDS formulation persisted for the development of NV AMD but not for central geographic atrophy.9 These results are consistent with the original recommendations that persons with intermediate or advanced AMD in one eye should consider taking the AREDS formulation.
However, people who smoked and took the beta-carotene containing AREDS formulation had a significantly higher risk of lung cancer than expected.
Another study, AREDS2, began in 2006. Chew and colleagues compared the beta-carotene formulation to one with 10 mg lutein and 2 mg zeaxanthin instead.11 At the end of the five-year study period, the researchers concluded that lutein and zeaxanthin did not increase the risk for lung cancer.
One of the conclusions from the AREDS 2 study is that the lutein/zeaxanthin combination was an appropriate and effective substitute for the original formula. Formulations based on the AREDS studies are readily available over the counter.
Treatment of geographic atrophy-associated dry macular degeneration
The FDA recently approved (February 2023) intravitreal injection pegcetacoplan for treating geographic atrophy-associated dry macular degeneration.12 The regulatory approval is based on positive data from the Phase III OAKS and DERBY trials conducted across a broad and representative population of GA patients.
Syfovre (brand name for pegcetacoplan) is a C3 and C3 inhibitor. This complement inhibitor decreases inflammation, phagocytosis, and cell membrane disruption. The eventual benefit would be to reduce retinal cell death and associated geographic atrophy.
Reduction of geographic atrophy was shown in the OAKS and DERBY studies. It also showed increasing effects of treatment over time, with up to 36% lesion growth reduction occurring between months 18 and 24. However, wet AMD is a potential adverse effect of pegctacolan use.
Iveric Bio company also has an injectable product (avacincaptad pegol) for geographic atrophy. This is a C5 complement inhibitor that met its primary endpoints in the GATHER2 study. This product is up for possible FDA approval in August 2023.
Up until recently, viable treatments have been limited to the less common wet form of AMD. Now that there are current and developing therapies for both types, it is essential for eye care practitioners to get up to date with these new and existing therapies. There is renewed hope for the millions of people suffering from AMD and we have an important role to discuss these new developments with our patients.
Written by: Dr David Jupiter, Optometrist
References:
- Pennington KL, DeAngelis MM. Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors. Eye Vis (Lond). 2016;22;3:34. doi:10.1186/s40662-016-0063-5
- American Macular Degeneration Foundation. Types and Stages of Macular Degeneration. Retrieved April 1, 2023 from https://www.macular.org.
- Barak Y, Heroman WJ, Tezel TH. The past, present, and future of exudative age-related macular degeneration treatment. Middle East Afr J Ophthalmol. 2012 Jan;19(1):43-51. doi: 10.4103/0974-9233.92115. PMID: 22346114; PMCID: PMC3277024.
- Argon laser photocoagulation for neovascular maculopathy. Five-year results from randomized clinical trials. Macular Photocoagulation Study Group. Arch Ophthalmol. 1991 Aug;109(8):1109-14. Erratum in: Arch Ophthalmol 1992 Jun;110(6):761. PMID: 1714270.
- Donati G, Kapetanios AD, Pournaras CJ. Principles of treatment of choroidal neovascularization with photodynamic therapy in age-related macular degeneration. Semin Ophthalmol. 1999;14:2–10.
- Schlötzer-Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt-Erfurth U. Dose-related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefes Arch Clin Exp Ophthalmol. 2002;240:748–57.
- Chakravarthy, U., Armendariz, B.G. & Fauser, S. 15 years of anti-VEGF treatment for nAMD: success or failure or something in between?. Eye 36, 2232–2233 (2022). https://doi.org/10.1038/s41433-022-02153-9
- National Eye Institute. (2022, April 2). NIH study confirms benefit of supplements for slowing age-related macular degeneration. Retrieved April 2, 2023 from NIH study confirms benefit of supplements for slowing age-related macular degeneration | National Eye Institute.
- Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Kurinij N, Davis MD; Age-Related Eye Disease Study Research Group. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. Ophthalmology. 2013 Aug;120(8):1604-11.e4. doi: 10.1016/j.ophtha.2013.01.021. Epub 2013 Apr 10. Erratum in: Ophthalmology. 2016 Dec;123(12 ):2634. PMID: 23582353; PMCID: PMC3728272.
- Age-Related Eye Disease Study 2 (AREDS2) Research Group; Chew EY, Clemons TE, Sangiovanni JP, Danis RP, Ferris FL 3rd, Elman MJ, Antoszyk AN, Ruby AJ, Orth D, Bressler SB, Fish GE, Hubbard GB, Klein ML, Chandra SR, Blodi BA, Domalpally A, Friberg T, Wong WT, Rosenfeld PJ, Agrón E, Toth CA, Bernstein PS, Sperduto RD. Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014 Feb;132(2):142-9. doi: 10.1001/jamaophthalmol.2013.7376. PMID: 24310343; PMCID: PMC4636082.
- Chew EY, Clemons TE, Agrón E, Domalpally A, Keenan TDL, Vitale S, Weber C, Smith DC, Christen W; AREDS2 Research Group. Long-term Outcomes of Adding Lutein/Zeaxanthin and ω-3 Fatty Acids to the AREDS Supplements on Age-Related Macular Degeneration Progression: AREDS2 Report 28. JAMA Ophthalmol. 2022 Jul 1;140(7):692-698. doi: 10.1001/jamaophthalmol.2022.1640. PMID: 35653117; PMCID: PMC9164119.
- Apellis. Syfovre was evaluated in two robust Phase 3 clinical trials with broad and heterogenous GA populations. Retrieved April 2, 2023 from https://syfovreecp.com/syfovre-clinical-trial-program.
- Liao DS, Grossi FV, El Mehdi D, Gerber MR, Brown DM, Heier JS, Wykoff CC, Singerman LJ, Abraham P, Grassmann F, Nuernberg P, Weber BHF, Deschatelets P, Kim RY, Chung CY, Ribeiro RM, Hamdani M, Rosenfeld PJ, Boyer DS, Slakter JS, Francois CG. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology. 2020 Feb;127(2):186-195. doi: 10.1016/j.ophtha.2019.07.011. Epub 2019 Jul 16. PMID: 31474439.
- Iveric Bio. Our Trials. Retrieved April 2, 2023 from Ivericbio.com/clinical-programs/#our-trials.
- Jaffe GJ, Westby K, Csaky KG, Monés J, Pearlman JA, Patel SS, Joondeph BC, Randolph J, Masonson H, Rezaei KA. C5 Inhibitor Avacincaptad Pegol for Geographic Atrophy Due to Age-Related Macular Degeneration: A Randomized Pivotal Phase 2/3 Trial. Ophthalmology. 2021 Apr;128(4):576-586. doi: 10.1016/j.ophtha.2020.08.027. Epub 2020 Sep 1. PMID: 32882310.

MDForLives is a vibrant community of healthcare professionals and patients dedicated to shaping the future of healthcare. We provide valuable global insights to healthcare companies through online surveys, interviews, and discussion forums.