Current treatment for schizophrenia typically relies on antipsychotic medications, but these drugs are not fully effective for all patients. Recently, an investigational drug combination called KarXT met its primary outcome in a Phase 3 schizophrenia trial.
KarXT consists of xanomeline and trospium chloride. The drug is being developed by Karuna Therapeutics. The results from the clinical trial could point to a new class of drugs for the treatment of schizophrenia.
Schizophrenia diagnosis and symptoms
Schizophrenia is a serious mental illness that can affect patients’ perception of reality, daily function, and overall quality of life. The disease symptoms are distressing and disruptive for patients and can also negatively impact relationships with friends and family as well as employment. Schizophrenia is often diagnosed after the first episode of psychosis between the ages of 16 and 30.
Symptoms of schizophrenia can vary between patients, but they fall into three categories: psychotic or positive symptoms such as hallucinations and delusions, negative symptoms such as loss of motivation or difficulty in showing emotions, and general cognitive symptoms such as problems associated with attention, concentration, and memory. These categories are the basis of the Positive and Negative Syndrome Scale (PANSS), which is used in the clinic to assess symptom severity in schizophrenia alongside clinical observation and chart data.

Caption: Schizophrenia affects patients’ perception of reality, their feelings, and their behaviors.
Treatments for Schizophrenia
Antipsychotic drugs that are commonly used to treat symptoms of schizophrenia work primarily by dampening the action of D2 dopamine receptors, while some also affect serotonin receptors. Many of these drugs are associated with significant adverse events, such as extrapyramidal symptoms, sedation, and weight gain, which can lead to the discontinuation of treatments and relapse of psychosis.
In addition, antipsychotics often do not affect the negative symptoms of schizophrenia and an estimated 20% to 33% of patients do not respond to standard antipsychotic treatments.
The muscarinic acetylcholine receptor (mAChR) has been of interest to drug developers because there is evidence suggesting that the muscarinic cholinergic signaling pathway may be disrupted in schizophrenia. However, the abundance of mAChR in the peripheral tissues makes it challenging to target the pathway specifically in the central nervous system (CNS) without causing side effects elsewhere in the body.
History of Xanomeline
The therapeutic component of KarXT is xanomeline, an M1/M4 mAChR agonist that was first developed by Lilly in the 1990s for the treatment of patients with Alzheimer’s disease (AD). AD patients undergoing the treatment reported reduced behavioral symptoms and psychosis.
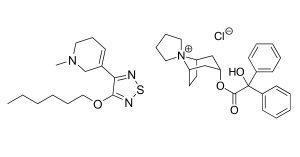
Caption: KarXT contains both xanomeline (left) and trospium chloride (right).
However, the drug’s development was later stopped due to high discontinuation rates due to adverse events such as gastrointestinal side (GI) effects associated with peripheral activation of mAChR. KarXT includes trospium chloride, an antagonist of mAChR that does not enter the CNS. Trospium is currently FDA-approved for the treatment of the overactive bladder.
Thus, in the drug combination, trospium chloride mitigates the side effects of xanomeline by blocking mAChR activation in peripheral tissues. A previous phase I trial found that the combination caused 50% fewer adverse events in healthy volunteers compared to treatment with xanomeline alone.
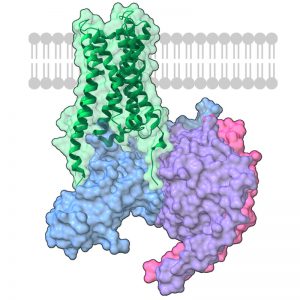
Caption: M1 mAChR is one of two muscarinic acetylcholine receptors that Xanomeline targets.
KarXT Clinical Trial
In early August 2022, Karuna Therapeutics announced the completion of the Phase 3 EMERGENT-2 Trial of KarXT.
In this double-blind trial, 252 people with schizophrenia and symptoms of psychosis were randomly assigned to either orally dosed KarXT or placebo twice daily for 5 weeks. The trial met its primary endpoint, with a statistically significant 9.6-point reduction in the PANSS total score in the treatment group compared to the placebo.
One potentially important finding is that, based on secondary endpoint data, the drug combination affected negative as well as positive symptoms of schizophrenia. Negative symptoms tend to be less well-controlled by existing medications.
Patients in the trial reported mild-to-moderate GI side effects and discontinuation rates were similar for both KarXT vs placebo (25% and 21%).
Karuna Therapeutics is looking to submit a New Drug Application for KarXT by mid-2023. Currently, the company has several Phase III trials in progress, which include investigating KarXT as a monotherapy or adjunctive therapy for schizophrenia and investigating its effects on psychosis in AD patients.

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.






