Patients with diabetes are at risk of cardiovascular disease. Heart failure, myocardial infarction, and stroke are all more common in individuals with diabetes, and cardiovascular diseases are the most prevalent cause of death in diabetics.1,2 Therefore, there has been great interest in learning how to improve cardiovascular health in patients with diabetes.
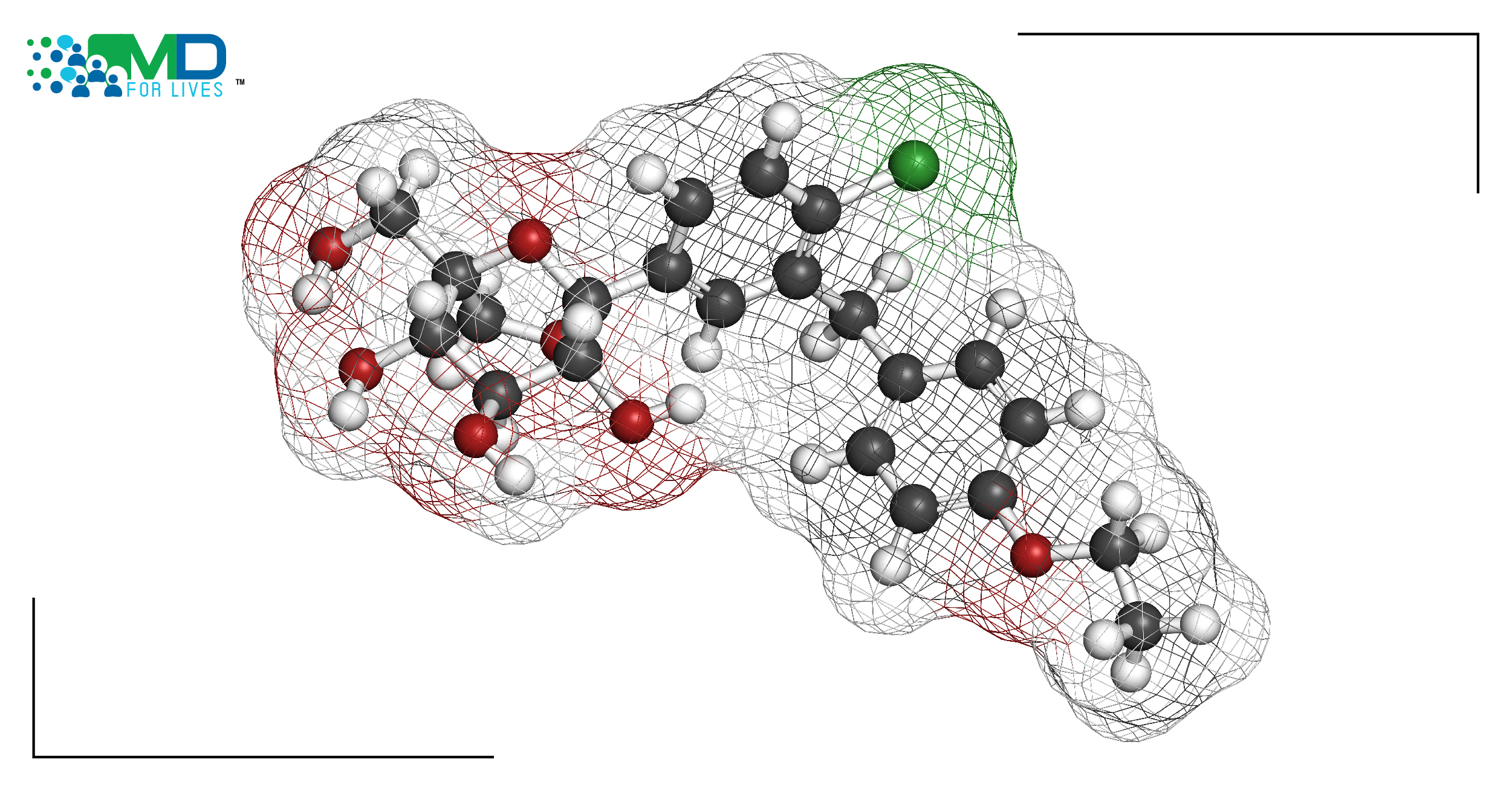
Sodium-glucose cotransporter 2 (SGLT2) inhibitors, also called gliflozins, are currently used in diabetes treatment for their ability to lower blood glucose. Since 2013, there has been extensive research to understand the potential uses of SGLT2 in protecting cardiovascular health as well. Now, clinical trial results have been reported for the combined SGLT1 and SGLT2 inhibitor sotagliflozin, which is currently approved for certain diabetes patients in the EU but not in the US.3

In the recent trials, researchers investigated the potential of sotagliflozin for the prevention of cardiovascular events in patients with type 2 diabetes. The heart failure clinical trial, SOLOIST-WHF, enrolled patients with both diabetes and recent worsening heart failure.4 The other trial, SCORED, included patients with both diabetes and chronic kidney disease.5 Both trials met their primary endpoints related to the prevention of cardiovascular events.
Gliflozins reduce the reabsorption of glucose in the proximal tubules within the kidneys, causing more glucose to be excreted in the urine, which, in turn, lowers blood glucose levels. Back in 2015, scientists noticed that these drugs can also reduce cardiovascular health risk, and several possible mechanisms underlying these effects are being studied.6 Large studies have supported the beneficial cardiovascular health effects of these drugs in patients with diabetes, and with or without existing heart disease.3 Since then, several practice guidelines have been published with recommendations on how to improve cardiovascular health in patients with diabetes using gliflozins.3,7

Several SGLT2 inhibitors are on the market in the US. The recent trials reported in the New England Journal of Medicine on January 14, 2021, investigated the use of a newer SGLT2, sotagliflozin, which targets both SGLT1 (in the gastrointestinal tract) and SGLT2 (in the kidneys). In April 2019, the EMA approved sotagliflozin for use in the EU in certain patients with type 1 diabetes. However, sotagliflozin comes with an increased risk of diabetic ketoacidosis, and the US FDA refused to approve the drug because of its safety issue.8
SGLT2 inhibitors appear to reduce cardiovascular risks in patients with stable heart failure.3,8 The SOLOIST-WHF trial analyzed whether sotagliflozin can lower cardiovascular risks in patients with diabetics and recent worsening heart failure. In this trial, 608 patients were randomly assigned to the sotagliflozin group and 614 to the placebo group. In sotagliflozin group, the drug did lower the composite of cardiovascular-related deaths, hospitalizations, and urgent visits to medical care, thus meeting the primary endpoint. The hazard ratio was 0.67. When considering just cardiovascular deaths, the hazard ratio was 0.84.9 Notably, the drug appeared to be effective in both patients with preserved ejection fraction (HFpEF) and those with reduced ejection fraction (HFrEF).3

The SCORED trial found that sotagliflozin lowered cardiovascular events in patients with both diabetes and chronic kidney disease who were considered to be at risk for cardiovascular disease. A total of 10,584 patients were enrolled, and one-half of the patients were randomized to the sotagliflozin group and the other half to the placebo group.10
The SCORED trial (as well as the SOLOIST-WHF trial) ended early because the sponsor withdrew funding, which resulted in a change to the primary endpoint for SCORED during the trial. The new primary endpoint, a composite of cardiovascular-related deaths, hospitalizations for heart failure, and urgent visits for heart failure, showed a statistically significant reduction in the sotagliflozin group with a hazard ratio of 0.74.10 Safety results from the two trials were consistent with the safety profile for the drug class based on from studies with other gliflozins.11




4 Comments
A Blood Glucose-Lowering Drug with the Potential to Protect Cardiovascular Health – PharmaCite
5 years ago[…] A Blood Glucose-Lowering Drug with the Potential to Protect Cardiovascular Health […]
Chronic Kidney Disease (CKD): Can it be Reversed? | MDforLives Blog
5 years ago[…] Early detection, evaluation, and management can prevent/slow the progression- can prevent kidney damage and cardiovascular diseases. […]
Dapagliflozin approved for use in chronic kidney disease - MDforLives
5 years ago[…] developing end-stage kidney disease.3 More recently, the authors of the SCORED trial reported that the SGLT2 inhibitor sotagliflozin reduced the number of cardiovascular events in patients who had both diabetes and CKD and who were at risk for cardiovascular […]
Can the Stages of Chronic Kidney Disease (CKD) be Reversed? - MDForLives
4 years ago[…] Early detection, evaluation, and management can prevent/slow the progression- can prevent kidney damage and cardiovascular diseases. […]