One of the greatest advancements in public health throughout the past century has been lauded as the development of vaccines. Other infectious organisms, such as fungi, have so far faced significant challenges in the creation of safe and effective vaccines, in part because of our limited understanding of the processes behind protective immunity.
The Growing Concern Around Fungal Pneumonia
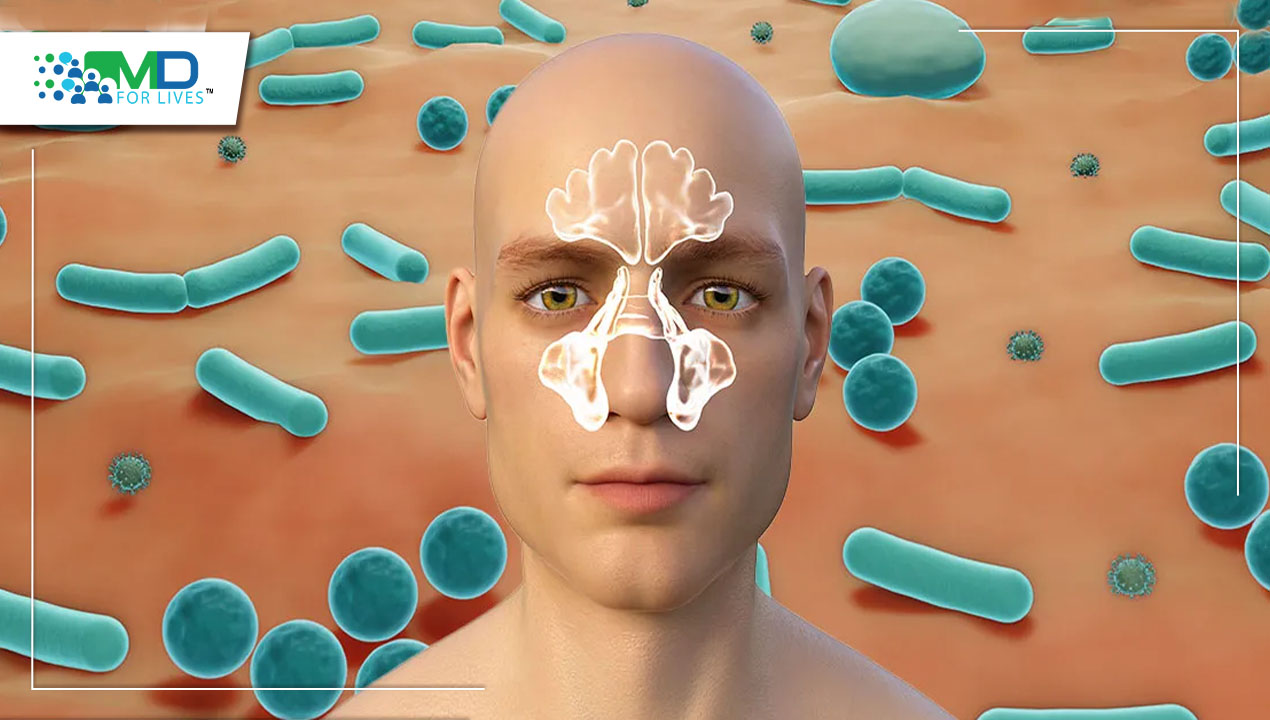
Fungal diseases are epidemiological markers of certain populations of individuals who are at risk, both in terms of their underlying health and the range of diseases they acquire. Fungi can induce severe, life-threatening types of infection in patients with significant immunological impairment, even though they can also produce lung symptoms and cutaneous lesions in people who appear to have a functioning immune system. In the past several decades, both the frequency of fungal illnesses and the number of immunocompromised people as a result of aggressive chemotherapy, solid organ or bone marrow transplantation, and autoimmune disorders have increased.
Common Fungal Pathogens Responsible for Fungal Pneumonia
Even though there are thousands of different fungus species, only a small number of them may harm people. Candida species, Aspergillus fumigatus, Cryptococcus neoformans, Pneumocystis jiroveci (carinii), and thermally dimorphic fungi such as Histoplasma capsulatum, Blastomyces dermatitidis, Coccidioides posadasii, Penicillium marneffei, and Paracoccidioides brasiliensis are the most prevalent fungus.
Advancements in Fungal Vaccine Research
A specific type of T cell, one of the body’s powerful immune defenses, is shown by researchers at the University of Illinois College of Veterinary Medicine to create cytokines that are required for the body to develop protection against fungus infections in a mouse model. This discovery may help in the creation of brand-new, efficient fungus vaccines. No vaccines have been approved to prevent or treat human fungal infections, even though vaccinations are heralded as one of medicine’s greatest innovations and are responsible for reducing or eliminating several infectious illnesses that are fatal. This deficiency was particularly fatal during the COVID-19 epidemic. Patients with underlying illnesses such as uncontrolled diabetes who were COVID-19 patients exhibited a higher risk of having deadly fungal infections in nations where steroids were often used to decrease pulmonary inflammation.
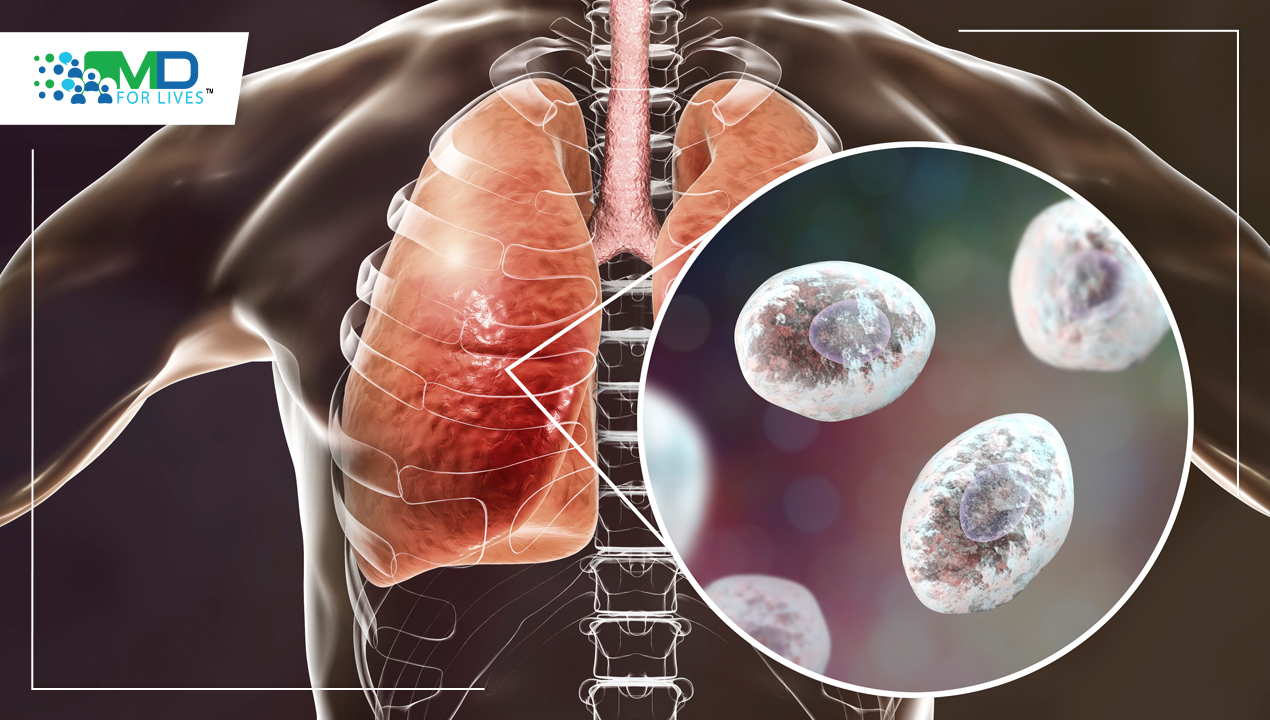
How can Fungal Pneumonia Develop?
In developed nations, pneumonia is the most common infectious cause of mortality. Only a tiny fraction of nosocomial and community-acquired pneumonia are caused by the fungus, which represent a minor portion of the wide variety of respiratory pathogens. However, the growing population of patients who are immunosuppressed is concerned about fungal respiratory infections. If fungi are a real pathogen, they can cause a wide range of clinical symptoms while invading body places without causing illness. A number of endemic or opportunistic fungi can cause fungal pneumonia, an infectious condition that affects the lungs. Following the inhalation of spores, conidia, or by the reactivation of a latent infection, fungal infection can happen. Hematogenous dispersion commonly takes place, particularly in a host with weakened immunity.
Use of CD8+ T Cells to Treat Invasive Fungal Infections
Patients with impaired immune systems experience significant rates of morbidity and death from invasive fungal infections (IFIs). Innate immune cells’ pattern-recognition receptors identify fungal infections and launch the initial line of defense against fungal infection. The adaptive immune system, which mostly consists of CD4+ T cells but also includes CD8+ T cells, is the second line of defense. Clinical studies for CD8+ T cell-based IFI vaccines are presently being conducted, and their usage may be particularly significant for individuals with acquired immune deficiency syndrome. The FDA has not yet authorized any of the vaccinations used to treat IFI. Here, researchers discuss present and upcoming CD8+ T cell-based antifungal immunotherapy techniques. They present recent developments in the management of IFIs with T cells generated with a Sleeping Beauty vector. Chimeric antigen receptor (CAR) T-cell therapy has shown highly encouraging outcomes in recent clinical studies for the treatment of leukemia patients. They postulated that IFI may likewise be managed using CAR T cells. Therefore, utilizing the extracellular region of Dectin-1, which binds to -glucan, we created a CAR that specifically targets -glucan, a sugar molecule present in most fungal cell walls.
Register Now and Share Your Experience by Participating in Paid Patient Survey!!
T Cells: Balancing Pathogenic and Protective Responses
According to Dr. Som Nanjappa, an assistant professor of immunology at the University of Illinois, a specific type of T cell [TH17 cells] that expresses GM-CSF [granulocyte-macrophage colony-stimulating factor] was linked to a greater severity of illness in people infected with the virus that causes COVID-19. This research demonstrates that GM-CSF-expressing IL-17A+ CD8+ T cell (Tc17) is required for mediating fungus vaccination immunity without causing hyperinflammation. It is so abundantly obvious that the antigen specificity of T lymphocytes, namely, whether they target viral, fungal, or bacterial pathogens, greatly influences whether they play a beneficial or negative function. Cell Reports published a paper on October 25 titled “GM-CSF+ Tc17” stating that cells are necessary to augment vaccination protection against fatal fungal pneumonia without producing overt disease.
The study’s coauthors are Dr. Nanjappa, Jaishree Sharma, a graduate student in the Department of Pathobiology, and Miranda D. Vieson, a clinical associate professor in the Department of Pathobiology and a board-certified veterinary pathologist in the college’s Veterinary Diagnostic Laboratory. Srinivasu Mudalagiriyappa is a former graduate student who is now a scientist with Insmed Incorporated, a global biopharmaceutical.
Developing Vaccine Immunity Against Fungal Pneumonia
A test-tube fungi vaccine was administered to mouse colonies in the research. The mice were then exposed to a dangerous fungus that might have killed them. Researchers might establish that GM-CSF+ Tc17 cells are required to promote vaccination protection. They also discovered that GM-CSF+ Tc17 cells need IL-1 and IL-23 cytokines to respond to the vaccination. For these cells’ long-term memory homeostasis, IL-23 is not necessary, but it is crucial for vaccination protection against pulmonary fungal infection.
The discovery of a useful T cell subset for fungus vaccine protection in this work supports attempts to create a vaccine platform with sufficient adjuvants to potentiate such a T cell subset. Accordingly, scientists have discovered a functional phenotypic marker that might be used to improve this subgroup in order to increase vaccination effectiveness, stated Dr. Nanjappa. His current NIH-R01 award will allow him to continue working on this approach for a fungus vaccine.
Read About
Prostate Cancer Treatment – A Recent Study
Hurler Syndrome Gene Therapy – A Recent Study

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.