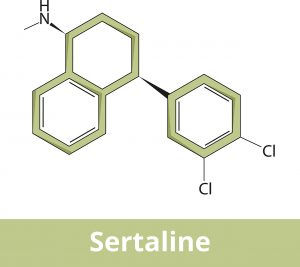
Sertraline is an antidepressant medication that is used to manage and treat major depression, obsessive-compulsive disorder, panic disorder, post-traumatic stress disorder, premenstrual dysphoric disorder, and social anxiety disorder.
Sertraline is taken orally once a day, in the morning or evening. If sertraline causes somnolence in the patient, it is given in the evening. When taken with food, sertraline absorption may be improved. Oral tablet dosages are 25 mg, 50 mg, and 100 mg, capsule dosages are 150 mg and 200 mg, and solution dosages are 20 mg/ml.
Discontinuation of serotonergic antidepressants may result in adverse effects, especially if the discontinuation is abrupt. Diaphoresis, dysphoric mood, irritability, agitation, vertigo, sensory disturbances (e.g., paresthesia, electric shock sensations), tremor, anxiety, confusion, cephalgia, lethargy, emotional lability, sleep disorder, hypomania, tinnitus, and seizures are among the symptoms. As a result, whenever possible, it is preferable to reduce the dosage gradually rather than abruptly.
Sertraline comes with a warning for children, teenagers, and adults under the age of 24. Sertraline may increase suicidal thoughts or actions, according to the study. Any family history of suicide should be disclosed to doctors by parents and caregivers. It is also critical to recognize warning signs of suicidal thoughts or behavior. Adults may also have suicidal thoughts during the first month or two of taking sertraline, as well as when the dosage is increased by the doctor.
Drug-drug Interaction
Sertraline and certain medications can interact. Interactions can result in unwanted side effects or medications that are more or less potent than intended. People should avoid taking monoamine oxidase inhibitors (MAOIs), another type of antidepressant, while taking sertraline or for up to two weeks after stopping. Taking sertraline with other serotonin-affecting drugs may increase the risk of serotonin syndrome. This syndrome is characterized by a variety of symptoms, including agitation and loss of muscle coordination.
Serotonin-affecting drugs include:
- Triptans
- Some other antidepressants
- Some pain relievers, such as tramadol
- Linezolid
- Pimozide
When taking medications like ibuprofen, warfarin, or aspirin, extreme caution should be used. Sertraline may increase the risk of bleeding.
Mechanism of action of Sertraline:
Sertraline is an antidepressant medication that belongs to the class of selective serotonin reuptake inhibitors (SSRIs). Sertraline works primarily by inhibiting presynaptic serotonin reuptake. Serotonin accumulates as a result of the inhibition of serotonin reuptake. Serotonin regulates mood, personality, and wakefulness in the central nervous system, which is why blocking serotonin reuptake is beneficial in disorders such as major depression. Sertraline also has little effect on norepinephrine and dopamine uptake, and studies have shown that it has more dopaminergic activity than other SSRIs. Sertraline’s mechanism of action makes it extremely effective in the treatment of a variety of psychiatric conditions.
The PANDA trial on the Effectiveness of Sertraline
The PANDA trial, a pragmatic, multicenter, double-blind, placebo-controlled randomized trial of patients from 179 primary care surgeries in four UK cities (Bristol, Liverpool, London, and York), included patients aged 18 to 74 years with depressive symptoms of any severity or duration in the previous 2 years and clinical uncertainty about the benefit of an antidepressant.

Patients were randomly assigned (1:1) to sertraline or placebo using a remote computer-generated code and were stratified by severity, duration, and site using random block length. Patients were given one capsule (sertraline 50 mg or placebo orally) per day for one week, then two capsules per day for up to 11 weeks, in accordance with the evidence on optimal dosages for efficacy and acceptability. The primary outcome was depressive symptoms measured by the Patient Health Questionnaire, 9-item version (PHQ-9) 6 weeks after randomization. Depressive symptoms and remission (PHQ-9 and Beck Depression Inventory-II), generalized anxiety symptoms (Generalized Anxiety Disorder Assessment 7-item version), mental and physical health-related quality of life (12-item Short-Form Health Survey), and self-reported improvement were secondary outcomes at 2, 6, and 12 weeks.
At 6 weeks, sertraline did not result in a clinically significant reduction in depressive symptoms. The mean 6-week PHQ-9 score in the sertraline group was 798 (SD 563) and 876 (586) in the placebo group. Sertraline, on the other hand, reduced anxiety symptoms, improved mental (but not physical) health-related quality of life, and self-reported mental health improvements. At 12 weeks, researchers found weak evidence that sertraline reduced depressive symptoms. They recorded seven adverse events, four for sertraline and three for placebo, and there was no difference in adverse events based on treatment allocation. Three serious adverse events occurred, two in the sertraline group and one in the placebo group.
Sertraline is unlikely to reduce depressive symptoms in primary care within 6 weeks, but researchers did observe improvements in anxiety, quality of life, and self-rated mental health, which are likely clinically significant. The findings support the use of SSRI antidepressants in a broader population than previously thought, including those with mild to moderate symptoms who do not meet diagnostic criteria for depression or generalized anxiety disorder.

Adverse effects of Sertraline
- SSRIs, a newer class of antidepressants, are considered to be more tolerable than tricyclic antidepressants or monoamine oxidase inhibitors. Sertraline’s most common side effects are syncope, lightheadedness, diarrhea, nausea, sweating, dizziness, xerostomia, confusion, hallucinations, tremor, somnolence, impotence, ejaculation disorder, fatigue, rhinitis, and female sexual disorder.
- Sertraline has a risk of causing bleeding because it inhibits platelet aggregation.
- Sertraline has the ability to prolong the QT interval; however, the prolongation is dose-dependent and very minor. Furthermore, citalopram has a higher risk of this than sertraline or other SSRIs.
- Sertraline may cause symptoms of serotonin syndrome in rare cases, but this is more likely when combined with another serotonergic medication. Myoclonus, muscle rigidity, diaphoresis, tremor, hyperreflexia, agitated delirium, and hyperthermia are among the symptoms.
- Sertraline, like other antidepressants, has been linked to an increase in suicidal ideation and behavior in children, adolescents, and young adults suffering from major depression.
- Sertraline should be used with caution in patients 65 years of age and older. The Beers Criteria classifies it as a high-risk medication in geriatric patients because it can cause a syndrome of inappropriate antidiuretic hormone or hyponatremia.
- Sertraline use during the first trimester of pregnancy increases the risk of cardiovascular malformations in infants, such as atrial and/or ventricular septal defects.
Sertraline exposure in neonates late in the third trimester has been linked to complications that necessitated prolonged hospitalization, tube feeding, and respiratory support.

Although sertraline has a number of benefits in treating anxiety and other mental disorders, its adverse effects cannot be disregarded.
Reference Links:
- https://www.ncbi.nlm.nih.gov/books/NBK547689/
- https://www.medicalnewstoday.com/articles/325632#drug-interactions
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7029306/

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.