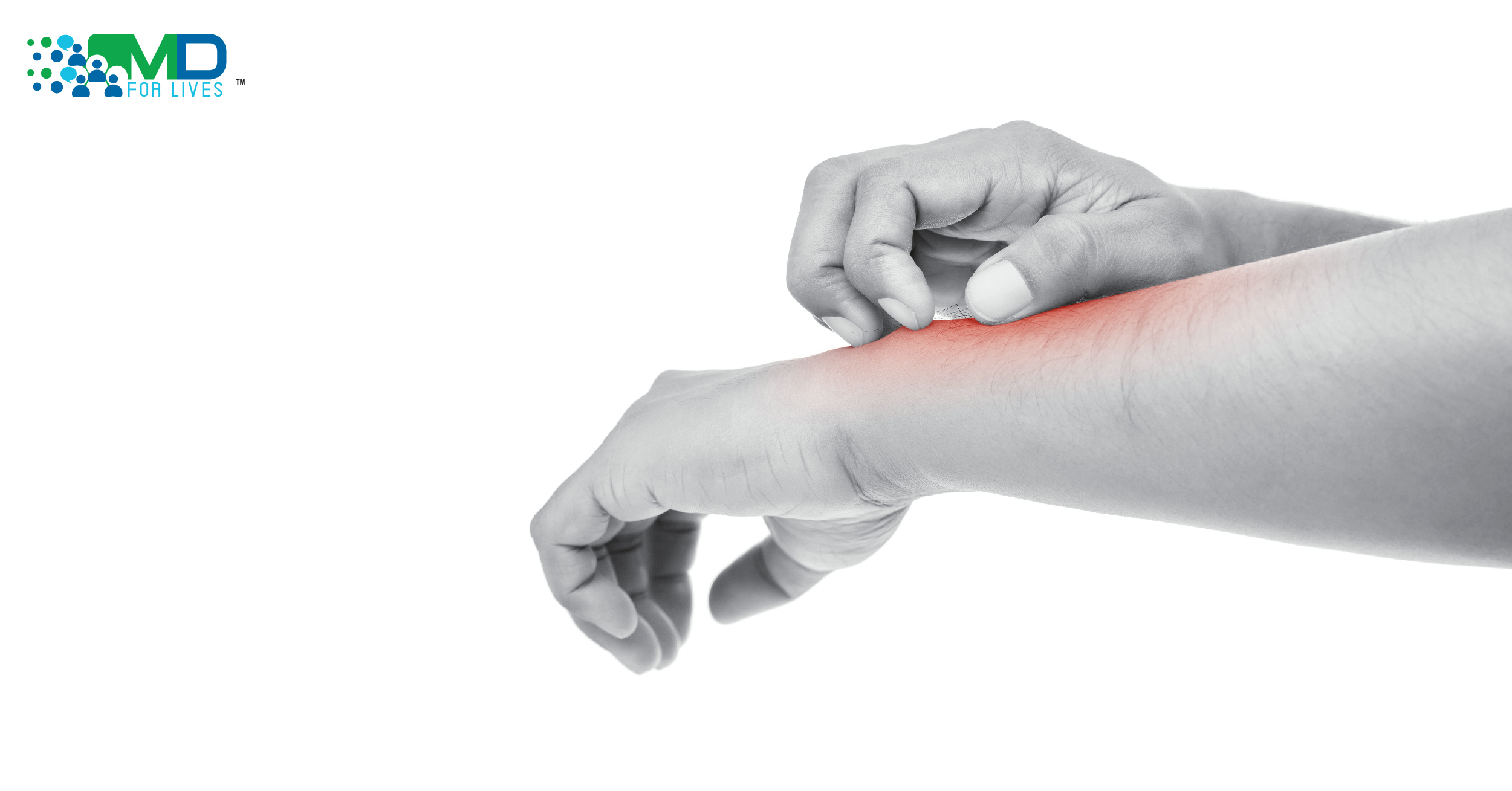
Atopic dermatitis, often called eczema, affects people of all ages. It is a chronic inflammatory skin condition that primarily results from increased production of pro-inflammatory cytokines, particularly interleukin (IL)-4 and IL-13, and involves Janus kinase 1 (JAK-1) pathway signaling. On March 25, 2021, researchers published Phase 3 clinical trial results for the JAK-1 inhibitor abrocitinib in the treatment of atopic dermatitis.ie, Atopic dermatitis therapy.
Current Treatments for Atopic Dermatitis
Historically, treatment for moderate-to-severe atopic dermatitis has included topical corticosteroids, calcineurin inhibitors, and phosphodiesterase 4 (PDE4) inhibitors. The monoclonal antibody dupilumab, an interleukin-4 receptor alpha antagonist, was approved in 2017 for patients with moderate-to-severe atopic dermatitis aged 6 years and older.
Abrocitinib: Therapy Under Study for Atopic Dermatitis
JAK-1 is a tyrosine kinase required for cytokine signaling. The oral JAK-1 inhibitor abrocitinib selectively inhibits IL-4 and IL-13 signaling (Figure 2) and is a direct target for therapeutic intervention of atopic dermatitis. It is currently under investigation in several clinical trials.

Phase 3 Clinical Trial on Abrocitinib
Data from the latest phase 3 trial (JADE-Compare) were published in the New England Journal of Medicine in March 2021. In this trial (NCT03720470, N=838), at weeks 12 and 16, abrocitinib (both 100mg and 200 mg daily oral doses) led to significantly greater reductions in signs and symptoms of moderate-to-severe atopic dermatitis than placebo. At week 2, the higher (200-mg dose) abrocitinib was superior to dupilumab in improving scores on an itch scale.
Patients with atopic dermatitis who did not respond to topical agents or needed systemic therapy were randomized to receive oral abrocitinib once daily (n=226 for 200 mg group and 238 for the 100 mg group), subcutaneous dupilumab (n=243; 300 mg every other week following a loading dose of 600 mg), or placebo (n=131).
The primary endpoints were improvement of ≥2 points from baseline on the Investigator’s Global Assessment (IGA, range:0-4, 0 [clear], 1 [almost clear]) and ≥75% improvement from baseline in Eczema Area and Severity Index (EASI-75, score range 0-72) at week 12. Secondary endpoints included itch response (improvement of ≥4 points in the Peak Pruritus Numerical Rating Scale [score range: 0-10]) at week 2 and IGA and EASI-75 responses at week 16.
At week 12, the investigators saw an IGA response in 48.4%, 36.6%, 36.5%, and 14.0% of patients in the abrocitinib (200 mg), abrocitinib (100 mg), dupilumab, and placebo groups respectively (P<0.001 for each of the abrocitinib doses vs. placebo). The investigators observed an EASI-75 response at week 12 (secondary endpoint) in 70.3%, 58.7%, 58.1%, and 27.1% of patients respectively (P<0.001 for each of the abrocitinib doses vs. placebo).
Adverse events included nausea (11.1% and 4.2% in the 200 mg and 100 mg dose groups respectively), and acne (6.6% and 2.9%, in the two groups respectively). At week 16, the results of the other secondary endpoints were similar for abrocitinib and dupilumab.
In addition to the JADE-Compare study, results of two earlier phase 3 studies, JADE MONO-1 and JADE MONO-2, have met primary and secondary endpoints. The JADE-Compare trial did not show any new safety signals.
Recent Clinical Trials for Abrocitinib
Recent, ongoing clinical trials for abrocitinib in moderate to severe atopic dermatitis include:
- Efficacy and safety in subjects aged 12 years and over, with the option of rescue treatment in flaring Subjects (NCT03627767 phase 3, actual primary completion date: September 2, 2020; actual enrollment: 1235)
- Comparison with dupilumab in adults on background topical therapy (NCT04345367 phase 3, estimated primary completion date: July 14, 2021; actual enrollment: 774)
- Efficacy and Safety with or without topical medications in subjects aged 12 years and older (NCT03422822 phase 3 [JADE extend], estimated primary completion date: December 1, 2023; estimated enrollment: 3000)
- Mechanism of action of PF-04965842 monotherapy (NCT03915496 JADE MOA phase 2, estimated primary completion date: September 6, 2021; estimated enrollment: 51)
- Expanded access protocol in adolescents and adults with moderate to severe disease (NCT04564755)
In late 2020, FDA granted priority review to abrocitinib for use in atopic dermatitis; the FDA’s decision is expected in quarter 3 of 2021.
Are you an HCP? Earn by Sharing your Insights! Register Now to Participate in Dermatology Survey
Also read