Biologics have long represented the pinnacle of pharmaceutical innovation. Developed through biotechnological processes, these have transformed the management of chronic diseases such as rheumatoid arthritis, inflammatory bowel disease, and various forms of cancer. With their unparalleled specificity and targeted mechanisms of action, biologics has improved clinical outcomes and redefined standards of care across specialties. For years, they stood largely unchallenged, protected by patent exclusivity, backed by robust clinical data, and priced at a premium reflective of their complexity.
However, the expiration of patents for many blockbuster biologics has opened the door to a new era. And this “new era” we talk about, is increasingly shaped by biosimilar drugs. These agents, designed to match their reference biologics in efficacy, safety, and quality, have begun to redefine pharmaceutical competition.
Supported by stringent regulatory frameworks in both developed and emerging markets, biosimilars drugs are rapidly gaining traction. Their promise of comparable clinical performance at a lower cost has positioned them as powerful tools to improve treatment access and reduce the financial burden on healthcare systems.
This paradigm shift has triggered important questions about the continued role and relevance of originator biologics.
To better understand the global medical community’s perspective, MDForLives engaged over 8,500 panelists (physicians) across five countries – the United States, United Kingdom, Germany, Italy, and Canada, to share their insights on the use, perception, and future of biologics and biosimilars.
Confidence Is Everything – Are Physicians Switching to Biosimilar Drugs?
When asked about their confidence in switching a stable patient from an originator biologic to a biosimilar, responses varied significantly across countries.
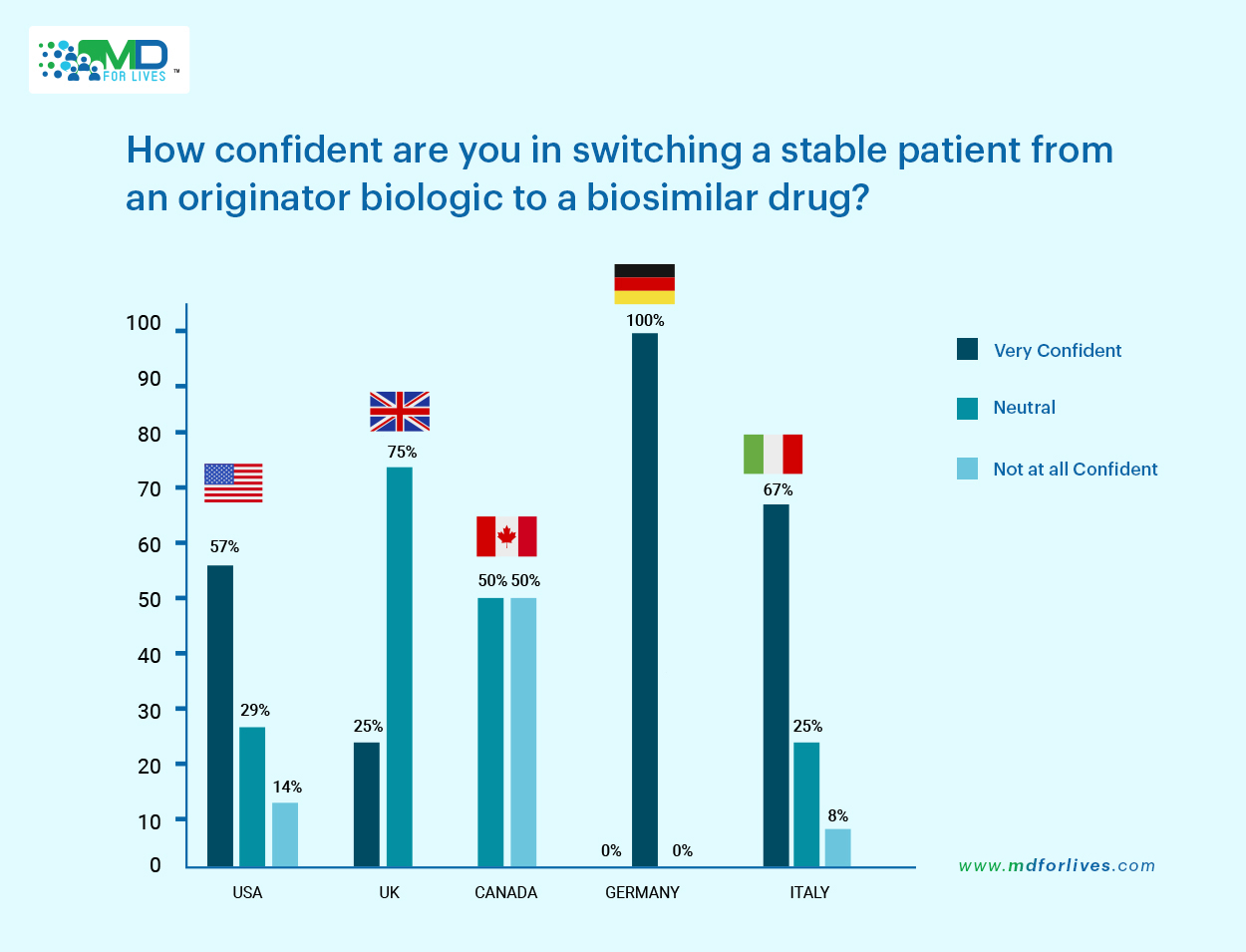
Italy (67%) and the United States (57%) stood out with the highest levels of confidence, suggesting a greater familiarity and trust in biosimilar drugs within these healthcare environments.
In contrast, a more neutral stance was observed in the UK (75%) and Germany (100%), where physicians appeared to adopt a cautious or “wait-and-watch approach”. Some of the notable reasons why we think that physicians in UK and Germany have probably remained neutral in case of biosimilar adoption is because:
- Biosimilars are relatively new, with limited long-term real-world data.
- Guidelines often lack clear direction on switching stable patients.
- Patients may resist switching due to trust in the original therapy.
- Physicians are cautious about disrupting effective, stable treatment.
However, what’s noteworthy is the level of concern reflected in Canada, where 50% of physicians reported they were not at all confident in switching stable patients to biosimilar drugs. The United States also showed some hesitation, with 14% of physicians expressing the same.
These results underscore the geographical differences in physician confidence and hint at varying degrees of familiarity and trust in biosimilars across healthcare systems.
‘Clinical Evidence’ and ‘Cost’ Lead the Conversation Around Biosimilar Adoption
Well, now that we are aware that most doctors from across the world are confident enough to switch to biosimilar drugs, naturally, we would like to know – why, or what made them switch.
When it comes to switching to biosimilars, physicians place the highest importance on clinical trial data, followed by cost-effectiveness.
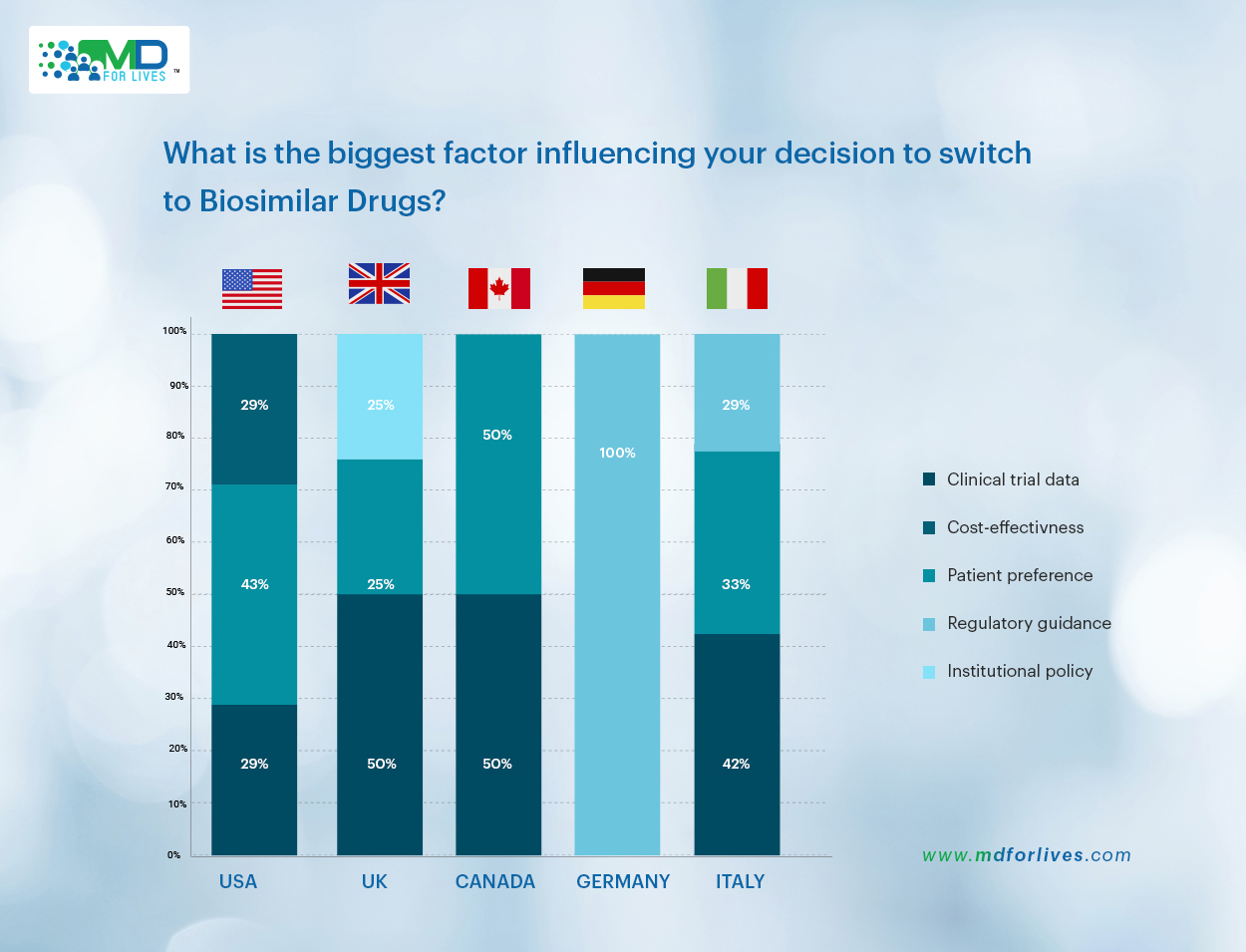
In Canada and the UK, both clinical evidence and cost were equally cited by 50% of respondents highlighting a balanced emphasis on efficacy and affordability. Italian physicians leaned slightly more toward clinical validation, with 42% prioritizing trial data over cost. In the United States, ‘cost-effectiveness’ emerged as the leading factor, selected by 43% of respondents, while clinical trial data and patient preference each influenced 29%.
However, in a striking contrast to other countries, regulatory guidance was the only factor influencing decisions in Germany (cited by 100 % of physicians surveyed).
Meanwhile, ‘Institutional Policy’ and ‘Patient Preference’ remained relatively minor influences across most countries. These insights reinforce that while cost considerations are gaining ground, scientific credibility continues to be the cornerstone of physician decision-making around biosimilars.
Switching to Biosimilars – A Transition Without Much Turbulence
Concerns about safety and tolerability often shape physician confidence in biosimilars. Encouragingly, half of all respondents reported never encountering any adverse events or clinical concerns when switching patients to biosimilars.
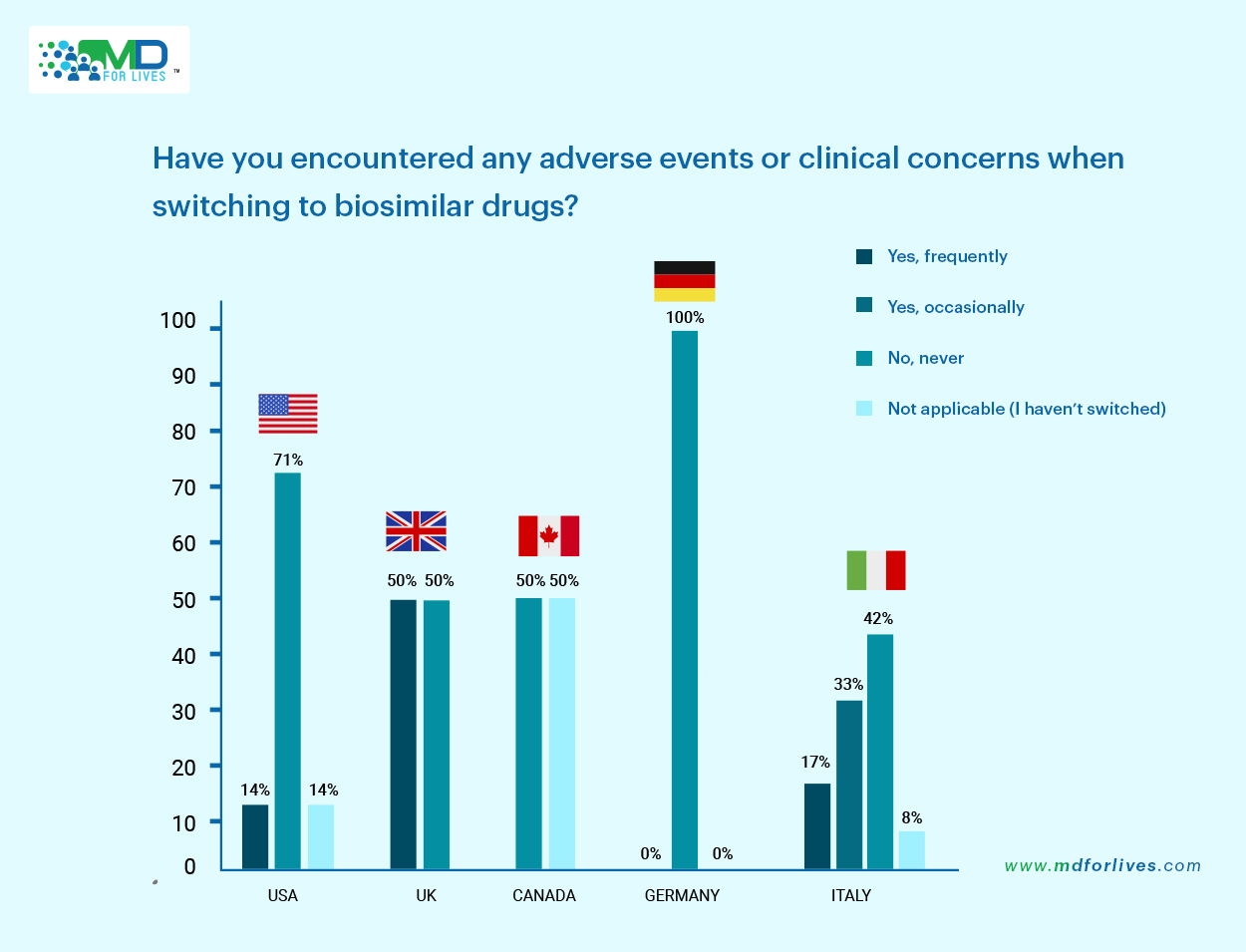
This sentiment was especially strong in Germany, where all physicians surveyed reported no issues, and in the United States, 71% shared the same view. While 31% of the global (total) respondents noted experiencing occasional issues, frequent adverse events were only reported by Italian physicians (17%).
Now, this could be attributed to several contextual factors:
- Italian physicians may have had earlier or broader exposure to biosimilar drugs in clinical practice, increasing the likelihood of encountering and reporting adverse reactions.
- Variability in product interchangeability guidelines, differences in pharmacovigilance practices, or even the type of biosimilars being prescribed could also play a role.
- Stronger reporting culture or heightened sensitivity to patient-reported outcomes in Italy might contribute to higher visibility of such events.
According to us, these points suggest just one thing – the need for a more localized investigation into biosimilar performance, physician training, and patient communication to ensure consistent confidence in switching practices across markets.
And when it comes to the overall insights, it is crystal-clear that while minor concerns do arise, the overall experience with biosimilar transitions has been largely positive.
All About the Last 12 Months – Biosimilar Adoption Rate
While biosimilars continue to gain ground globally, the pace and extent of adoption vary significantly across countries.
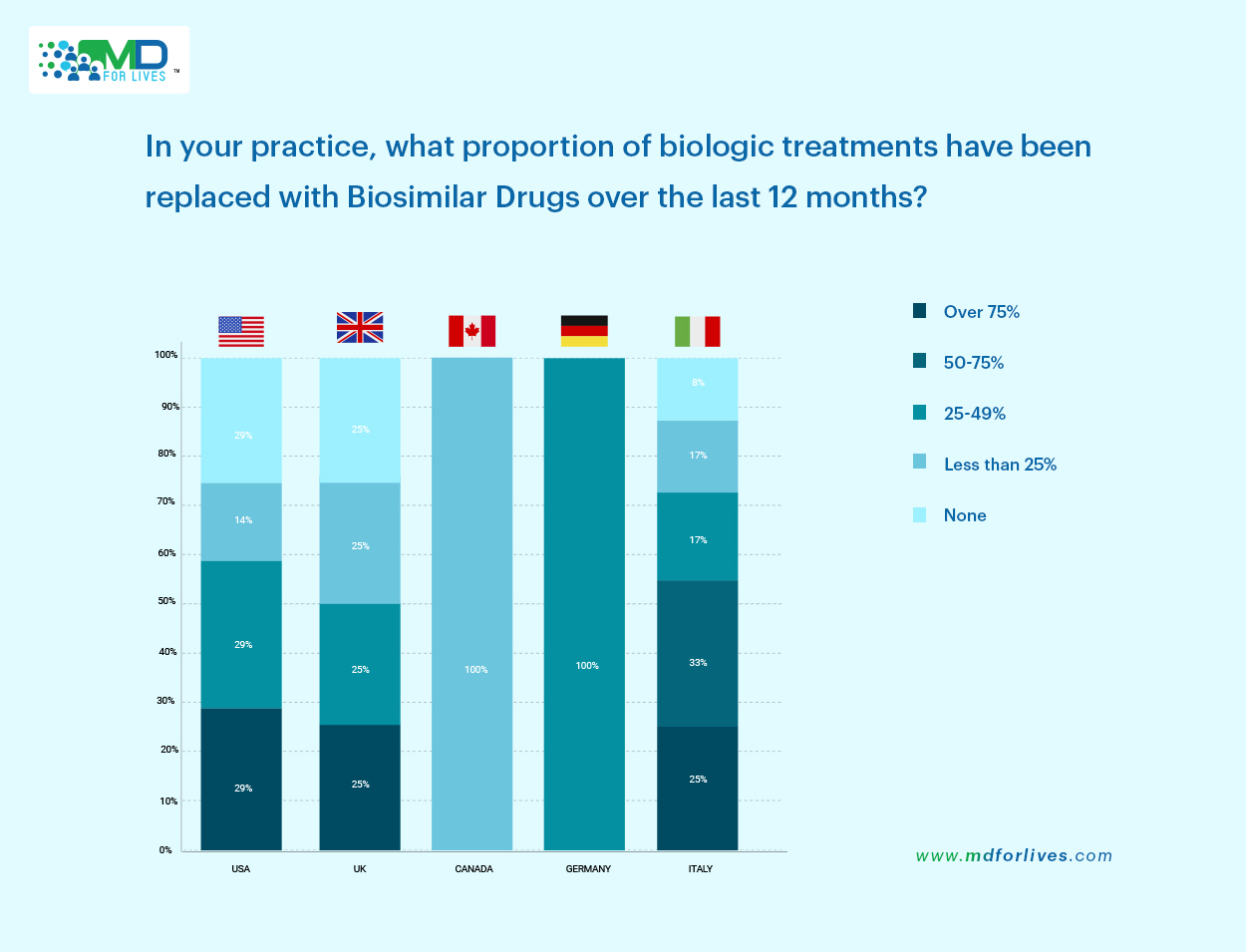
Italy demonstrated particularly high uptake, with 25% of physicians stating they had replaced over 75% of biologic drugs, and another 33% reporting replacement in the 50–75% range. This indicates strong integration.
German physicians on the other hand reported a full 100% replacement rate within the 50–75% bracket, signaling high confidence in biosimilar use.
In the United States, adoption patterns were more mixed, with 29% of physicians reporting both moderate (50–75%) and low (25–49%) levels of replacement. The United Kingdom followed a similar pattern.
But here’s the contrast – Canada appeared more cautious, with 100% of respondents stating that less than 25% of their biologics had been replaced with biosimilars.
After critically going through these insights, what we figured out is that these variations highlight the influence of national policies, institutional readiness, and physician comfort levels in driving biosimilar adoption over biologics.
Final Verdict
Our findings through this MDForLives survey show that physicians are steadily embracing biosimilar drugs, driven by strong clinical evidence, economic viability, and a desire to increase patient access. Yet, the battle between biologics and biosimilars is not one of elimination, but evolution. Biologics continue to command trust where stability, long-term data, and brand familiarity matter most – particularly in complex or high-risk cases.
What we are witnessing is not a clear winner or loser, but a rebalancing of the treatment ecosystem. Biosimilars are gaining territory, no doubt, but biologics drugs are not retreating. Instead, they are adapting, innovating, and in some cases, repositioning themselves for niche roles or next-generation advancements.
In the end, this is not about competition, but coexistence.
Share this insightful article on your social media handles if you find this interesting. Don’t forget to tag us @MDForLives.

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.