Clostridioides difficile is an important bacterial pathogen that sickens half a million Americans every year. This infection can be difficult to treat, often recurs after treatment, and can be fatal. Clostridioides difficile infection is associated with disruptions to the microbiota and often occurs after taking antibiotics for another infection. Two recent studies use our growing understanding of the human microbiome to illuminate paths to controlling this potentially deadly infection.
How does Clostridioides Difficile Infection Occur?

Clostridioides difficile bacteria seen under scanning electron microscopy (SEM).
Clostridioides difficile (formerly called Clostridium difficile) is an anaerobic, spore-forming bacterium that is carried by an estimated 2 to 3% of healthy adults and 20 to 40% of hospitalized adults. The spores can germinate when the gut microbial flora are disrupted, often after taking broad-spectrum antibiotics. Clindamycin exposure and long courses of antibiotics are especially associated with C. difficile infections.
The activated bacteria then produce toxins that cause colitis and diarrhea. Symptoms can range from mild to severe and can include diarrhea, abdominal pain, fever, pseudomembranous colitis, ileus, or toxic megacolon. Every year, approximately 29,000 people die from C. difficile in the US.

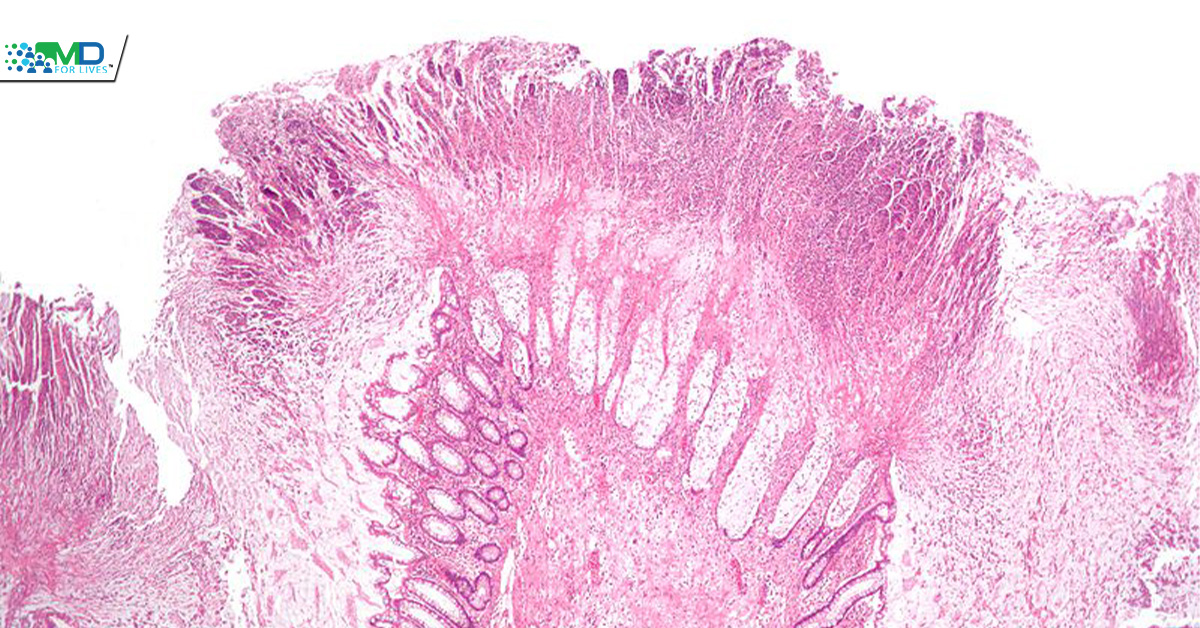
Pseudomembranous colitis is one of the potential effects of C. difficile infection.
Recurrent Clostridioides Difficile Infection is an Important Problem
Antibiotics (vancomycin and fidaxomicin) are used to kill the toxin-producing bacteria and resolve symptoms. However, antibiotics do not kill the spores, and the infection resurges in approximately 25% of patients after vancomycin treatment concludes. Some patients have multiple recurrences of C. difficile infections over the course of months, despite appropriate antibiotic treatment. When this occurs, the Infectious Diseases Society of America (IDSA) recommends transplantation of fecal microbiota from a healthy donor; this treatment has been associated with 68 to 90% remission rates in different studies. Bezlotoxumab, a monoclonal antibody against the C. difficile toxin, was approved by the FDA in 2016 for the prevention of recurrence in adults taking antibiotics for C. difficile infection. To prevent a recurrence, patients with recent C. difficile should also avoid taking antibiotics unless there is a clear need.
Researchers Apply Investigational Clostridioides Difficile Treatment
One recent publication reports the use of an investigational therapy that contains purified spores of beneficial bacteria to treat patients with recurrent Clostridioides difficile infection. Bacteria in the Firmicutes phylum are an important part of the healthy microbial flora. Some of these bacteria help defend the gut against pathogens, including by releasing metabolites that inhibit C. difficile spore germination. The researchers purified live bacterial spores from four stool donors, characterized the taxonomic composition of the mixture, and prepared oral capsules. Patients who had had three or more antibiotic-treated episodes of C. difficile infection were randomized to receive the bacterial therapy (n=89) or placebo (n=93).
The trial, which was supported by Seres Therapeutics, met its primary objective, with the bacterial therapy reducing the percentage of patients who had a recurrence of Clostridioides difficile infection within the 8-week follow-up period (12% vs. 40% with placebo). Adverse events were similar between the two groups, except that some adverse events were more common in the placebo group, and no serious adverse events or deaths were deemed treatment-related.

Another recent study showed that in mice, commensal bacteria can help modify C. difficile virulence, either increasing or decreasing rates of death and disease severity.
The researchers found that Paraclostridium bifermentans, an amino acid fermenter, reduced the virulence of C. difficile when mice raised in a sterile environment were colonized with both bacteria. By contrast, sterile-raised mice colonized by C. difficile and Clostridium sardiniense, a butyrate producer, developed more severe disease and died more quickly. Using conventional mice, the researchers then found that dosing C. difficile-infected mice with P. bifermentans bacteria could aid their survival.
Even with species that are considered commensal, this research points to the complexity of bacterial strains’ effects in the gut, especially when they interact with pathogens and other members of the microbiota. Both of these studies indicate promising future directions in treating the microbiome disruption associated with Clostridioides difficile infection.
Also Read
T Cells for Fungal Pneumonial Immunity