Dyslipidemias are a group of lipid metabolism alterations that manifest themselves with a variation in the concentration of plasma lipoproteins, a pathological condition representing one of the main risk factors for cardiovascular disease.
The guidelines recommend it as the standard therapy for managing dyslipidemia, the statins, but the residual CVD risk noticed in clinical practice has highlighted the importance and necessity of new therapy.
In addition to the possibility of treating these disorders with innovative drugs such as monoclonal antibodies and vaccines, emerging therapies on which it is essential to focus are siRNA (small interfering RNA) and ASOs (antisense oligonucleotides), which through gene silencing, offer valuable prospects for achieving a reduction in LDL cholesterol or alternative targets including triglycerides, Lp(a) and inflammation, while also offering the possibility of increased adherence to therapy.
These drugs have different mechanisms of action, but the target is common. One with a crucial and fundamental role in lipid metabolism, such as PCSK9, ApoB, Lp(a), ApoCIII, is chosen as the target.
Gene silencing is a post-transcriptional process of controlling the expression of a gene. ASOs and siRNAs through this method turn off a gene. This is not made available for translation into the corresponding protein, a protein involved in lipid metabolism or playing a crucial role in the pathogenesis of dyslipidemia.
ASO: what are they?
Antisense oligonucleotides, also called ASOs, are short, single-stranded nucleotide sequences of DNA or RNA (less than 20 bases) that need to be carried into the cytoplasm, where they bind specifically by pairing to an mRNA sequence, thereby inhibiting the synthesis of the corresponding protein.
VOLANESORSEN, a drug that has revolutionized the treatment of FCS through gene silencing
Volanesorsen (trade name Waylivra), a drug approved in Europe in March 2021, is a second-generation ASO.
Indicated as an adjunct to diet in adult patients with genetically confirmed familial chylomicronemia syndrome (FCS) and high risk of pancreatitis, in whom response to diet and triglyceride-lowering therapy has been inadequate. Effective treatment for familial chylomicronemia syndrome (FCS), a disease, inherited rare dyslipidemia that affects 1-2 in 1,000,000 people with a very severe impact on the patient leading to marked elevation of triglyceride levels, was long overdue.
This condition’s symptoms are physical and debilitating, such as abdominal pain, eruptive skin xanthomatosis, hepatosplenomegaly, and cognitive and emotional problems can also occur. The only possible treatment, until volanesorsen came on the market, was to lead a rigorous lifestyle and diet to reduce TG levels and, thus, the risk of pancreatitis. Therefore, the discovery and subsequent commercialization of this drug were significant.
Volanesorsen’s target is the mRNA of APOC3; it binds to it, thus promoting its degradation, consequently accelerating the catabolism of TGs.
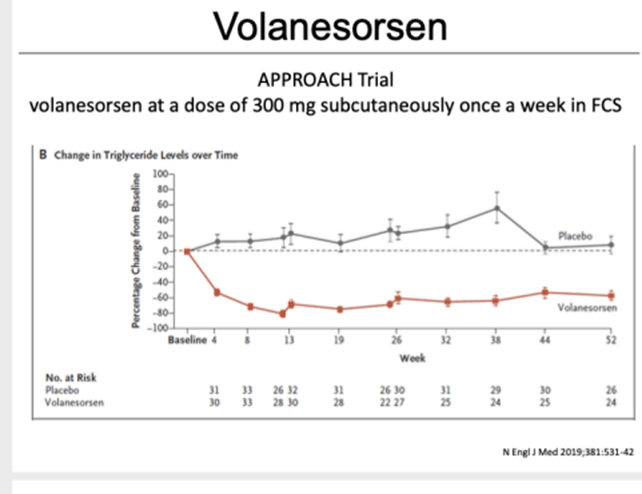
Figure 1 Mechanism of action of Volanesorsen, ASO binds the mRNA sequence of ApoCIII by blocking its transcription. Failure to produce ApoCIII leads to increased LPL activity and, consequently, reduced triglyceride levels
Volanesorsen is administered subcutaneously with a variable dosing regimen, generally requiring one subcutaneous administration per week for the first three months and then increasing to 2 administrations per month (every two weeks).
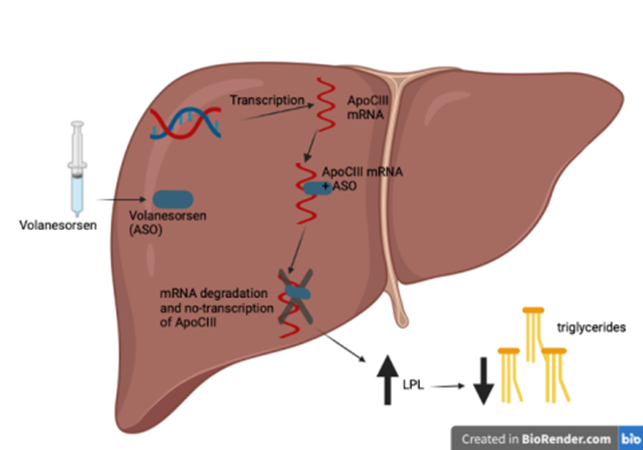
Figure 2 Volanesorsen at a dose of 300mg subcutaneously once a week in FCS. The image shows the change in ApoC3 level with the drug or the placebo.
Efficacy, safety, and tolerability have been extensively evaluated in several preclinical studies in animal models with different types of diets and phase I clinical trials (first clinical trial in 2015), where in healthy subjects, it produced a significant reduction in apoCIII and TG levels.
This favorable pharmacological profile supported further clinical investigations through phase II and III studies. Important phase III studies for this drug were: the approach, compass and broaden study.
APPROACH TO CLINICAL TESTS
This Phase III study evaluated the drug’s efficacy, which started in 2014 and ended in 2017.
For this study, 66 patients with FCS (genetically confirmed either by LPL activity < 20% or fasting TG levels 750 mg/ dL) were recruited.
Patients were randomized to take the drug for 52 weeks at a dosage of 300mg sc per week or placebo.
Primary outcome: Change in fasting triglyceride (TG) percentage at three months after dosing.
Secondary outcome: Frequency of acute pancreatitis episodes.
Results:
After 13 weeks of therapy (300 mg/week), plasma apoC-III levels were reduced by 84% from baseline and a reduction in TG levels of less than 750 mg/dl was recorded in 77% of patients treated with volanesorsen.
This reduction should be important, as 750 mg/dl is considered the threshold value that would significantly reduce pancreatitis.
TG values from baseline decreased by 76.5% compared with a 17.6% increase in the placebo group. This effect remained significant even after 6 and 12 months.
Regarding other outcomes, Volanesorsen also reduced levels of TG chylomicrons (-82.7%), apoB 48 (-75.9%) and VLDL-C (-58.3%).
The most common side effect was injection site reactions, most of which were mild to moderate, and reduced platelet counts in 16 patients.
Clinical trial 🡪 COMPASS
The COMPASS study is a randomized, double-blind, placebo-controlled phase 3 trial. This study aims to evaluate the efficacy and safety of volanesorsen administered for 26 weeks in participants with hypertriglyceridemia.
A total of 114 patients with hypertriglyceridemia (fasting TG values ≥500 mg/dL.) were recruited for this trial.
Primary outcome: reduction in TG levels.
Secondary Outcome: reduction in HDL-C levels and reduction from baseline glycated hemoglobin values in patients with type II diabetes mellitus.
Results:
Three months of volanesorsen (300 mg/once a week) reduced TG by 73% from baseline, maintaining a stable effect over 26 weeks.
The most common side effect was injection site reactions; no serious platelet events were reported.
In contrast, it is an important finding that in patients with type 2 diabetes, there was a reduced reduction in glycate levels, but a 42% increase in HDL-C was reported.
Combining the APPROACH and COMPASS studies data shows that nine pancreatitis events occurred in six placebo-treated patients compared with the only event in one volanesorsen patient.
BROADENING clinical trials
It is a randomized, double-blind, placebo-controlled phase 3 study evaluating the efficacy of volanesorsen in patients with familial partial lipodystrophy who have moderate to severe hypertriglyceridemia and elevated apoC-III levels. This study involved the weekly administration of 300 mg volansorsen to recruited patients.
Patients recruited:
Forty patients (11 males and 29 females) with FPLD (familial partial lipodystrophy is a disorder in which body fat is missing in various areas and to varying degrees, which can also lead to metabolic diseases such as insulin resistance, hepatic steatosis and dyslipidemia).
Primary outcome: Reduction in TG levels.
Secondary Outcome: Improvement of insulin resistance, diabetes, and hepatic steatosis.
Results:
There was an 88 percent reduction in triglyceride levels in the drug-treated patients at three months compared with a 22 percent reduction in placebo-treated patients. This lowering was maintained throughout the 12-month study period.
Additionally, liver fat was reduced by 51.9%.
This study observed that treatment with volanesorsen led to a significant reduction in ApoC3 levels associated with a reduction in insulin resistance, thus underscoring a correlation between the two.
To improve the efficiency of Volanesorsen, this has been conjugated with a GalNac moiety leading to the design of APOCIII-LRX (olezarsen), leading not only to increased specificity toward the target but also to an improved safety profile.
As a result of these studies, Volanesorsen appears to be very effective in achieving a significant reduction in TG levels, which we recall playing an additional role in the risk of cardiovascular events and the development of acute pancreatitis.
From the compass study, we infer that the drug may also be useful in reducing glycated hemoglobin values, a factor that may predispose to the development of diabetes mellitus. The use of this drug has also improved the quality of life of patients with FCS, who previously were forced to forego performing errands of daily living or work due to the debilitating symptoms associated with this condition.
Many of the patients treated with this ASO reported that they managed their symptoms better and made it possible, for example, to eat a fat-rich meal without having abdominal problems, which was previously unthinkable.
In addition to these benefits, data obtained from the trials support the central role of apo CIII as a modulator of the lipolytic cascade and its inhibition as an effective strategy for developing new drugs for hypertriglyceridemia.
Written by: Dr Lucrezia Stefanetti
References:
[1] “A new dawn for managing dyslipidemias: The era of RNA-based therapies”. G.- Macchi, C.R. Sirtori, A. Corsini.
[2] “Antisense oligonucleotides: absorption, distribution, metabolism, and excretion.” Mohammad Shadid, M.Badawi.
[3] “Familial chylomicronaemia syndrome”. L. D’Erasmo, A. Di Costanzo, M. Arca.
[4] “The APPROACH Study: A Study of Volanesorsen (Formerly IONIS-APOCIIIRx) in Patients with Familial Chylomicronemia Syndrome”. ClinicalTrials.gov
[5] “Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial“. Lancet Diabetes Endocrinol.
[6] “The BROADEN Study: A Study of Volanesorsen (Formerly IONIS-APOCIIIRx) in Participants with Familial Partial Lipodystrophy.” Clinicaltrials.gov

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.