Hodgkin’s lymphoma (HL) or Hodgkin’s disease is a cancer of the lymphatic system characterized by the uncontrolled expansion of irregular lymphocytes. As of 2022, it is estimated that there are 8540 new cases of HL and 920 related deaths per year in the US.
Advances in treatment within the last few decades have resulted in a 5-year survival rate of 87%, which is higher than that of many other types of cancer. However, treating advanced-stage patients remains challenging, especially considering that the initial treatment selection can influence survivorship.
Recently, long-term data were published from the Phase III clinical trial of an alternative therapy regimen for stage III and IV disease. The multinational trial with 1334 patients, one of the largest trials reported on this disease, found that the alternative therapy regimen led to better survival, at least in the study’s patient population, than that associated with current standard treatment.
What is Hodgkin’s Lymphoma?
HL is most commonly diagnosed in young people between the ages of 15 and 30 and in adults above 55. The two categories of HL are classic HL (CHL), which is the predominant disease type, and nodular lymphocyte-predominant HL (NLPHL). CHL is characterized by the presence of large multinucleated cells called Reed-Sternberg cells in various parts of the lymphatic system, especially the lymph nodes.
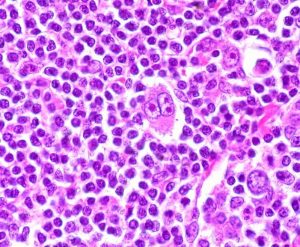
Caption: Hodgkin’s lymphoma is characterized by the presence of large and multinucleated Reed-Sternberg Cells.
Collection and buildup of the abnormal cells lead to tumor growth and a weakened immune system. Symptoms include enlarged lymph nodes, fever and/or night sweats, severe itching and fatigue. Risk factors for HL include infection with the Epstein-Barr virus/mononucleosis, age (early adulthood and late adulthood), gender (predominantly males), family history of HL and a weakened immune system.
Treatment for Hodgkin’s Lymphoma
The standard first-line chemotherapy is the ABVD regimen, a combination of doxorubicin hydrochloride (Adriamycin), bleomycin sulfate, vinblastine sulfate and dacarbazine. For early-stage disease, the National Comprehensive Cancer Network (NCCN) guidelines recommend 2 cycles of ABVD followed by interim positron-emission tomography (PET) scans before deciding on whether to intensify or de-escalate therapy.
The subsequent treatments can consist of more cycles of ABVD or radiation therapy. Patients cleared of positive PET scans after two cycles of ABVD often have a good prognosis and can stop therapy after two additional cycles.
The balance between efficacy and long-term toxicity must be considered for the treatment of patients who are at advanced-stage disease and do not meet the negative PET scan criteria. In this case, patients are treated with more rounds of ABVD or with the very intensive escalated BEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine and prednisone) regimen (4-6 cycles), which can cause serious toxic effects.
Adverse effects of these treatments include pulmonary toxicity, heart disease, second cancers and infertility. For patients above 65, no more than 2 cycles of bleomycin are recommended due to severe lung toxicity.
A+AVD and ABVD for Hodgkin’s Lymphoma
More recently, an alternative regimen, A+AVD, has become available. In A+AVD, bleomycin from the standard regiment is replaced with brentuximab vedontin, an anti-CD30 antibody-drug conjugate. As previously reported, the ECHELON-1 trial compared treatment with up to 6 cycles of A+AVD or ABVD and found a progression-free survival benefit for A+AVD in the trial’s patient population.
Now, six-year follow-up data from ECHELON-1 show an overall survival advantage (93.9% vs 89.4%) for the trial patients treated with A+AVD, an important milestone for doctors and patients deciding between the two treatments. In addition, fewer patients who received the A+AVD treatment received subsequent therapies compared to the ABVD group.
One important consideration for patients and doctors in choosing between A+AVD or ABVD is the cost, which can be more than $275,00 higher with the new regimen.
The two regimens’ different safety profiles must be weighed. A recent study reported more deaths due to pulmonary toxic effects and second cancers in the ABVD group. However, an editorial published in NEJM points out that the number of pulmonary toxicity-related deaths in the ABVD group was higher than would be expected with careful lung function monitoring, which could be due to variations in practice between the trial sites in 21 different countries. The A+AVD group reported a higher rate of patients with ongoing peripheral neuropathy.
Currently, the NCCN guidelines still recommend ABVD over A+AVD, except in specific situations.

MDForLives is a vibrant community of healthcare professionals and patients dedicated to shaping the future of healthcare. We provide valuable global insights to healthcare companies through online surveys, interviews, and discussion forums.