The HD-550 video endoscopy system from SonoScape has been approved by the US Food and Drug Administration
The FDA has authorized SonoScape’s flagship video endoscopy system HD-550 for gastrointestinal diagnostics, marking a significant step forward in SonoScape’s Endoscopic product roadmap. The HD-550 endoscopic equipment features a 4-LED light source that supports 1080P high definition and allows for multi-spectrum and multi-mode imaging. The chromoendoscopy SFI (Spectral Focused Imaging) and VIST (Versatile Intelligent Staining Technology) light modes, in addition to the high-performance white-light mode, improve vascular and mucosal color contrast. As a result, more information about the GI tract can be revealed, potentially assisting clinicians in the discovery, delineation, and characterization of lesions. The 550 series videoscopes have been cleared, and their maneuverability has been acknowledged by leading endoscopists all around the world.
New insights into lymphoid cell maturation may lead to more effective IBD treatments
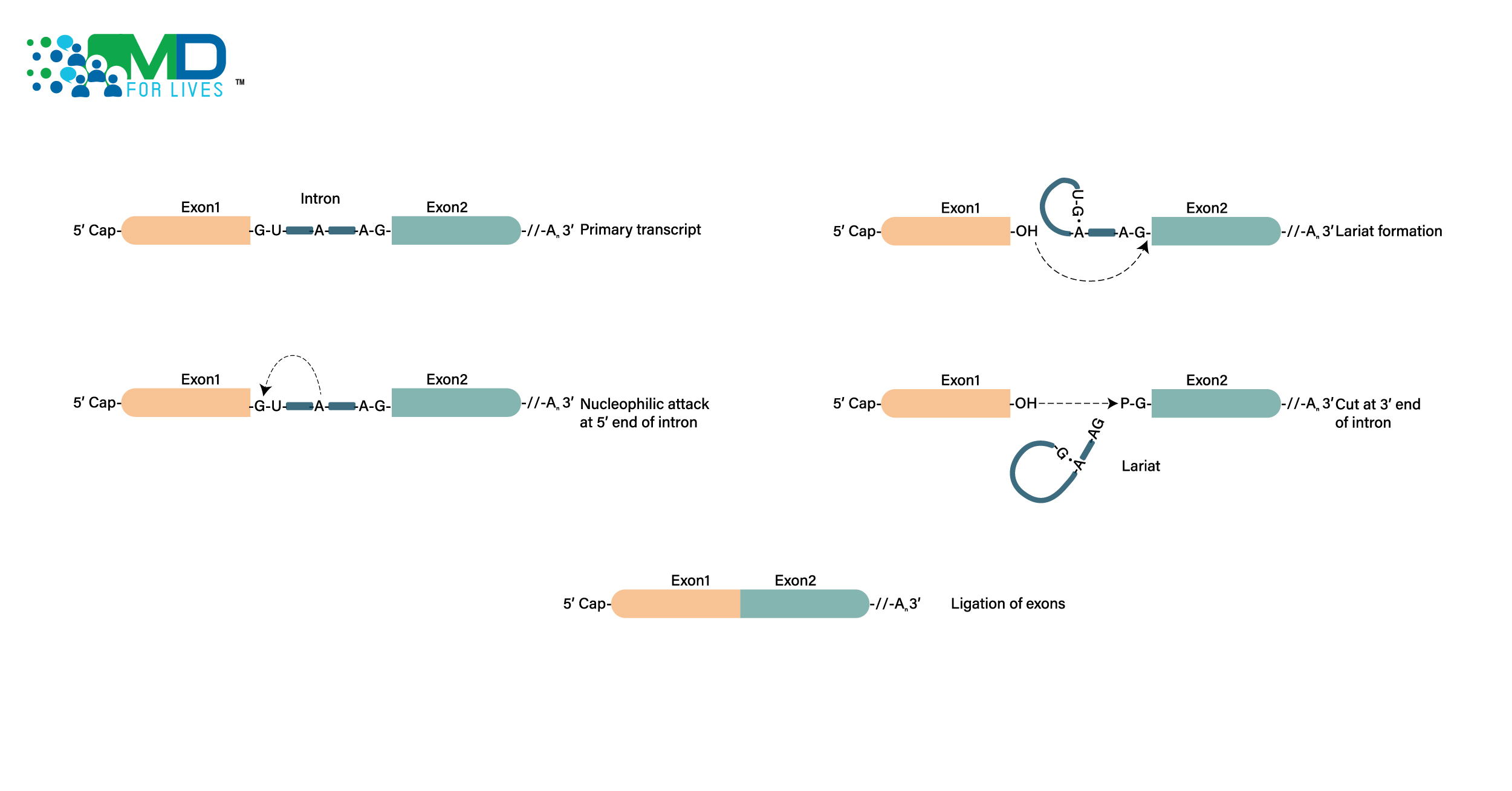
A team of researchers at Sweden’s Karolinska Institutet studied how innate lymphoid cells (ILCs), which play a role in inflammatory bowel disease, evolve into mature cells (IBD). The discoveries may pave the way for more effective treatments for IBD, a disease that causes a lot of pain and is associated with a higher risk of colon cancer.
Inflammatory bowel disease (IBD) is characterized by persistent inflammation of the gut mucosa, which has been linked to an increased risk of colon cancer. Symptoms such as stomach pain and weight loss commonly appear before the age of middle age.
The etiology is unknown; however, it is thought that genetic, environmental, and immunological factors all have a role. Because a considerable proportion of IBD patients do not react to current treatments, there is a pressing need for fresh research regarding the disease’s processes.
ILCs are lymphocytes, a type of immune cell found in the mucosa. ILCs have been found to modify function during inflammation in previous studies, making them a prospective target for IBD treatment.
ILCs were isolated from the tonsils and gut tissue of patients who had had resection surgery or endoscopic examination in this investigation. The trial included a total of 48 individuals, 31 of whom had IBD. Following that, the ILCs were thoroughly investigated, both immediately after isolation and after cell culture.
Bendit Technologies’ Bendit 021 steerable microcatheter has received 510(k) clearance from the US Food and Drug Administration to treat brain, peripheral, and coronary vasculature
Bendit Technologies stated that their Bendit21 microcatheter has gained FDA 510(k) clearance for treatment of the brain, peripheral, and coronary vasculature. The clearance came several months after the Bendit21 neuro catheter was successfully used in two life-saving surgeries in the United States.
A steerable distal tip is included with the Bendit21 steerable microcatheter, which is controlled by a ‘steering slider’ on the proximal steering handle. By twisting the torque knob on the steering lever, endovascular specialists can rotate the tip in both directions. These improved steering and torqueability maneuvering capabilities are designed to allow navigation through all vasculatures more precisely and correctly, with or without guide wires, potentially widening indications, improving safety, and reducing procedure time.
Emergex presents an update on its innovative dengue fever and coronavirus T cell adaptive vaccines’ first-in-human investigations
Emergex Vaccines Holding Limited offered an update on its dengue and coronavirus T cell adaptive vaccine candidates’ first-in-human clinical studies.
Emergex has introduced an innovative clinical trial design for its novel T cell adaptive vaccines, in which two vaccine candidates based on the same platform technologies, one against dengue fever and the other against coronavirus disease, are evaluated in two stages of phase I clinical trial with 52 participants at a single site in Switzerland (Unisanté, Lausanne).
The trial’s two rounds are aimed to assess the vaccine candidates’ safety and tolerability in a double-blind, randomized, dose-ranging, and comparator-controlled setting. The clinical trial has been overseen by an independent Data Safety Monitoring Board.
The business has completed the phase I clinical trial for its innovative CD8+ T cell dengue vaccine candidate, dubbed naNO-Dengue. The trial’s last participant’s last visit, as per the clinical trial protocol (NCT04935801), took place on March 11, 2022. In the study, 26 healthy adults aged 18 to 45 years old were given two dose levels of the Emergex CD8+ T cell dengue vaccine. Following the first injection, trial participants were followed for six months, and the final clinical trial report is nearing completion. In the summer, the results are expected to be released.
The company’s unique CD8+ T cell coronavirus vaccine candidate is currently in phase I clinical study (NCT05113862), with the first participant dosed on January 10, 2022. The ‘naNO-COVID’ experiment’s 26 healthy people aged 18 to 45 years have all been vaccinated against coronavirus disease with the Emergex CD8+ T cell vaccine (lower-dose or higher-dose) and are currently in the follow-up period, as per the clinical trial protocol.
The trial in Lausanne took place during the Omicron wave of SARS-CoV-2 in Switzerland, which occurred between January and March. The seven-day average of new cases reached 30-40,000 per day during this time period, suggesting a considerable rise over previous time periods. Participants are being evaluated for virologically verified symptomatic illness, and the trial remains blinded (comparator-controlled).
In HLA transgenic mice, preclinical data for the company’s novel CD8+ T cell coronavirus vaccine candidate confirmed that no lung inflammation was detected after vaccination with the company’s T cell coronavirus vaccine candidate and subsequent SARS-CoV-1 intranasal challenge, indicating that the vaccine candidate demonstrates heterologous protection for both viruses from the same family.
Emergex is a leader in the development of 100% synthetic T cell adaptive vaccines that use the body’s natural T cell immune response to destroy pathogen-infected cells to protect against some of the world’s most serious health threats, including viral infectious diseases like dengue, coronaviruses, and pandemic influenza, and serious intracellular bacterial infectious diseases.
http://www.pharmabiz.com/NewsDetails.aspx?aid=149112&sid=2
http://www.pharmabiz.com/NewsDetails.aspx?aid=149114&sid=2
http://www.pharmabiz.com/NewsDetails.aspx?aid=149144&sid=2
http://www.pharmabiz.com/NewsDetails.aspx?aid=149143&sid=2