Nonalcoholic steatohepatitis (NASH) is an often silent but dangerous liver disease that affects an estimated 2% to 5% of adults in the US. The availability of new liver disease treatments is important as NASH and related disorders are increasingly common in the US and worldwide. An article published in October 2021 reports positive results in a Phase 2b clinical trial of the investigational drug lanifibranor for the treatment of NASH. The drug targets all three peroxisome proliferator-activated receptors (PPARs) found in the human body; other PPAR agonists are used in the treatment of dyslipidemias, diabetes, and other conditions.4
What is Nonalcoholic Steatohepatitis?
Nonalcoholic steatohepatitis (NASH) is a disorder involving fat buildup and inflammation in the liver. It is related to but more severe than non-alcoholic fatty liver disease (NAFLD), which affects an estimated 25% of US adults. In addition to the steatosis (fat buildup within cells) and inflammation, which are hallmarks of the disorder, NASH can also involve damage to hepatocytes and progressive liver fibrosis, which can eventually lead to cirrhosis. Risk factors include obesity, diabetes or prediabetes, age over 40 years, and high blood triglycerides, among others.
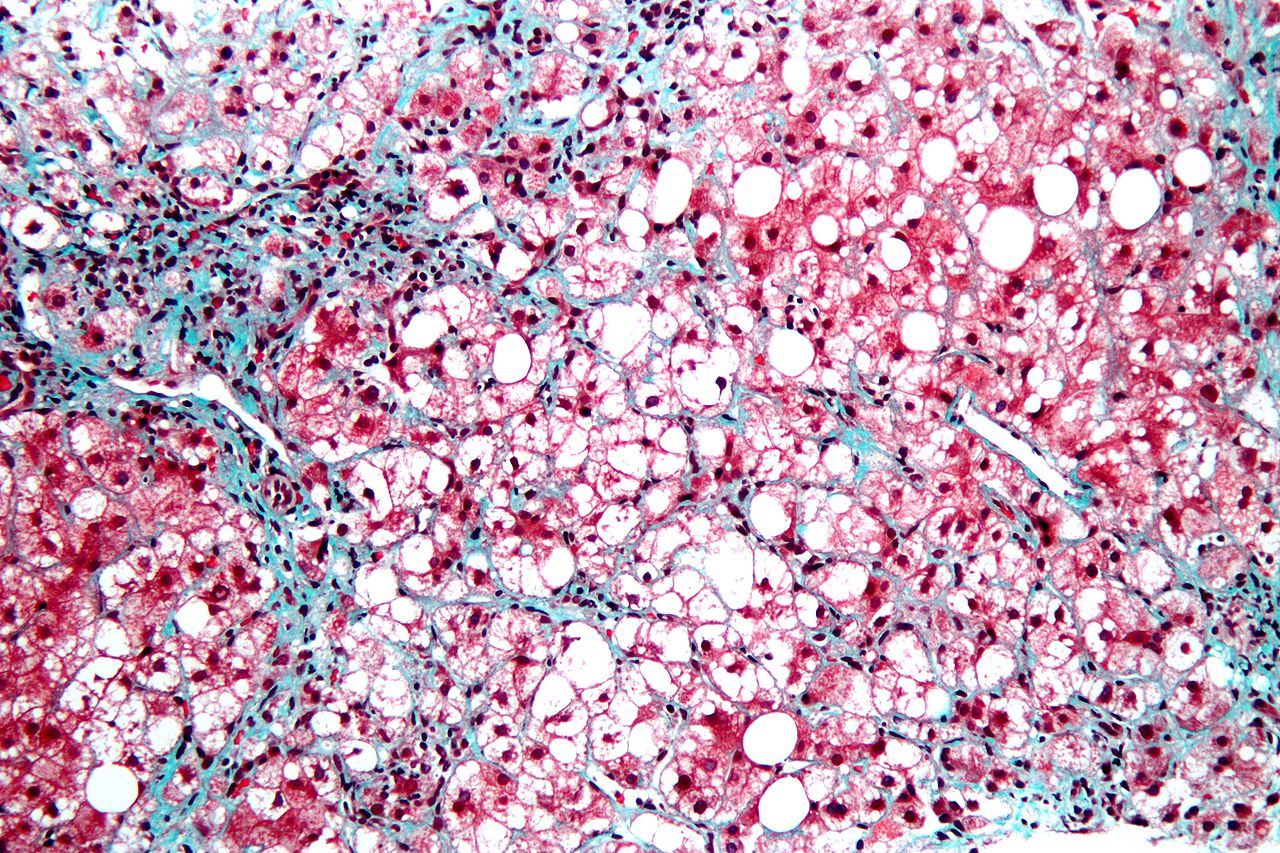
NASH liver disease symptoms are often subtle or nonexistent and can include fatigue and pain in the upper right side of the abdomen. The changes in the liver can resemble those in alcoholic fatty liver disease but are not caused by alcohol. NASH also needs to be distinguished from other causes of liver injury, such as exposure to hepatotoxic medications or chronic hepatitis C infection.

NASH Liver Disease Treatment
NASH can eventually progress to cirrhosis and/or liver cancer. However, the changes seen in NASH are at least partially reversible through lifestyle changes. Weight loss through diet and exercise is a key means of controlling NASH. Improving diabetes control, reducing cholesterol, and consuming less sugar and salt are also recommended. The FDA has not yet approved any medications specifically for NASH.
Trial of a Potential NASH Liver Disease Treatment
PPARs are nuclear receptor proteins that help regulate gene transcription, affecting energy and lipid metabolism as well as inflammation. Individual PPARs are targeted by the fibrates as well as the thiazolidinediones; there has been recent research into drug candidates that target two or all three of the PPAR isotypes.
- Saroglitazar, which targets the alpha and gamma subtypes, has been approved in India for the treatment of NASH and of dyslipidemia in patients with diabetes, but it is not approved in the US.
- The experimental drug lanfibranor is a pan-PPAR agonist, meaning that it targets all three of the PPAR subtypes in the human body.
- The Phase 2b lanifibranor trial, referred to as NATIVE, was conducted at multiple sites in North America, Europe, Australia, and Africa. This randomized, double-blind trial included 247 patients who were assigned to receive 1200 mg lanfibranor, 800 mg lanifibranor, or placebo, daily for 24 weeks.
The trial met its primary endpoint; a decrease of 2 or more points in the SAF-A score (which includes measures of hepatocellular ballooning and lobular inflammation) and no increase in the fibrosis stage from the baseline to week 24. The higher dose of lanfibranor provided significant improvements in disease manifestations over placebo, with 55% of patients (1200 mg) vs. 33% of patients (placebo) meeting the primary endpoint criteria, while the 800 mg dose did not prove as successful (48% of patients met primary endpoint criteria).
Inventiva, the company developing lanfibranor, is planning a phase 3 trial. Results are expected in 2024.
Also read
Growth Hormone Therapy for Obesity and Nonalcoholic Fatty Liver
Epclusa – Treatment for Hepatitis C infection