July is Hemochromatosis Screening and Awareness Month. In honor of this important event, this article will highlight recent research on hemochromatosis.
What causes high iron levels in Hemochromatosis?
Hemochromatosis is a disorder involving excessive iron buildup in the body. The excess iron can damage organs including the liver, heart, and joints, and if treatments fail, the disorder can be fatal.
Hemochromatosis can be genetic (primary hemochromatosis) or can be secondary to another disorder. Primary hemochromatosis is classified into four types, which vary in severity and age of onset. Type 1, also called “classic” hemochromatosis, affects around 1 million people in the US.
Normally, a protein called hepcidin regulates human iron metabolism, including the amount of dietary iron the body absorbs through the gastrointestinal tract. In individuals with Type 1 hemochromatosis, a mutation in the gene encoding hepcidin is responsible for the high iron levels. Types 2, 3, and 4 are associated with other genetic mutations.
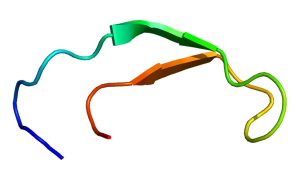
Structure of the hepcidin protein. (Creative Commons image by Emw, from https://en.wikipedia.org/wiki/Hepcidin#/media/File:Protein_HAMP_PDB_1m4f.png )
An important cause of secondary hemochromatosis is repeated blood transfusions. People with thalassemia, sickle cell disease, bone marrow failure, and certain other conditions may require repeated blood transfusions over months or years. Because the human body cannot excrete excess iron, transfusions gradually lead to a buildup of iron in the body. People with untreated anemia due to kidney failure and those with severe liver disease can also develop iron overload.
Hemochromatosis diagnosis and screening
Individuals who have a first-degree relative with hemochromatosis are recommended to receive genetic screening for the disorder.5 Further investigation is recommended for individuals who show possible symptoms of iron overload, as well as those who have abnormal results on transferrin saturation or serum iron tests.
Hemochromatosis diagnosis may include a medical and family history, a physical exam, genetic testing, and blood tests for levels of iron, transferrin, and ferritin, as well as the iron-to-ferritin ratio. If liver disease is suspected, a liver biopsy may be performed to confirm iron buildup in the liver.
Hemochromatosis symptoms can include joint pain, weakness, fatigue, abdominal tenderness, and “bronzing” or other changes in skin appearance. However, affected individuals may not show symptoms for years or decades.
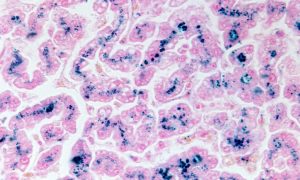
Iron deposits in hepatocytes (stained blue) can occur in hemochromatosis.
Current hemochromatosis treatment
Today, therapeutic phlebotomy (blood draws to remove iron from the body) is a mainstay of treatment for primary hemochromatosis. It is effective for most patients but cannot reverse organ damage that has already occurred. Chelation therapy is another important treatment, especially for those with secondary hemochromatosis, but it comes with toxicity issues and cannot prevent all the harms caused by iron overload.
Potential therapies for Hemochromatosis
Several experimental therapies are under investigation. In April 2022, Bond Biosciences began a Phase 1 clinical trial of an oral medication intended to bind dietary iron and prevent its absorption to prevent iron accumulation in people with hemochromatosis.
A synthetic mimic of hepcidin, called rusfertide, is currently in Phase 2 studies for potential use in primary hemochromatosis. Rusfertide, which is also in trials for polycythemia vera, was placed under an FDA clinical hold in the fall of 2021 due to the development of tumors in laboratory animals treated with the drug, but the hold was lifted a month later.
In earlier-stage research, scientists at Stanford University identified a possible future therapeutic strategy for iron overload cardiomyopathy — damage to the heart that is one of the most serious complications of hemochromatosis. In laboratory studies, they found that a molecule called ebselen lessened the amount of iron that entered cultured cardiomyocytes and also improved the function of iron-overloaded cardiac cells.6 Ebselen is currently in clinical trials for a number of other purposes, including ototoxicity and treatment of Meniere’s disease, but it is not currently FDA-approved for any condition.
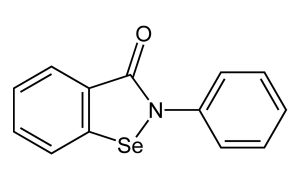
Ebselen structure. Public domain image from https://en.wikipedia.org/wiki/Ebselen#/media/File:Ebselen-2D-skeletal.png
References:
- https://irondisorders.org/july-is-national-hemochromatosis-screening-and-awareness-month/
- https://rarediseases.org/rare-diseases/classic-hereditary-hemochromatosis/
- https://medlineplus.gov/genetics/condition/hereditary-hemochromatosis/
- https://www.niddk.nih.gov/health-information/liver-disease/hemochromatosis/symptoms-causes
- https://emedicine.medscape.com/article/177216-guidelines
- https://med.stanford.edu/news/all-news/2020/09/researchers-find-potential-cure-for-deadly-iron-overload-disease.html
- https://www.niddk.nih.gov/health-information/liver-disease/hemochromatosis/treatment
- https://www.sciencedirect.com/science/article/abs/pii/S0378595521000435?via%3Dihub
- https://www.biospace.com/article/releases/bond-biosciences-announces-first-dosing-of-sentinel-patients-in-a-phase-ia-b-clinical-trial-for-a-novel-therapy-to-treat-patients-with-hemochromatosis/
- https://www.fiercebiotech.com/biotech/protagonist-s-story-gets-a-happy-ending-as-fda-s-hold-swiftly-lifted-rusfertide-program

MDForLives is a vibrant community of healthcare professionals and patients dedicated to shaping the future of healthcare. We provide valuable global insights to healthcare companies through online surveys, interviews, and discussion forums.