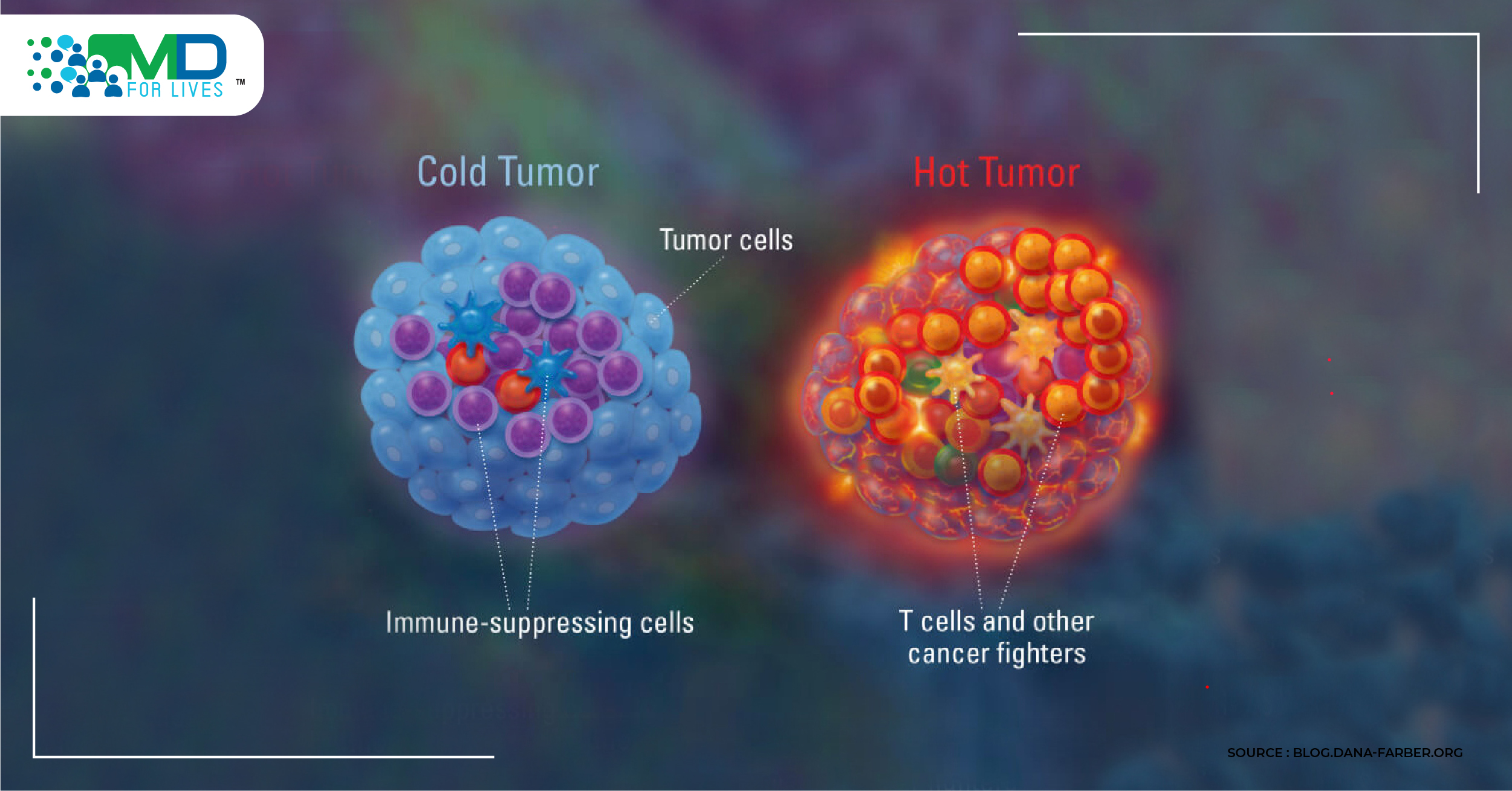
The field of immunology is transforming the landscape for cancer patients. When you can get a T-cell into the tumor, many of the immunotherapeutic approaches show their teeth, but why are there still a large percentage of patients who cannot get T-cells into the tumor? The goal of immunotherapy is, ostensibly, how do we turn a cold tumor hot?
This is where the oncolytic virus (OV) bulldozes its way in.
The oncolytic virus offers a number of important attributes:
- They are selectively cytotoxic to the tumor cells. We are at the beginning of understanding how a tumor cell dies, this knowledge will no doubt lead to an improvement in therapeutic approaches.
- Naturally, the oncolytic virus infection and oncolysis elicit innate and adaptive immune responses, which are required for long-term anti-tumoral immunity; at the same time, the viral infection prompts an anti-viral response.
- The oncolytic virus is very well tolerated as a class, with minimal side effects. A definite improvement on what cancer patients have had to tolerate the last few decades.
- Some oncolytic viruses are able to express various transgenes, allowing us to manipulate the immune system even further. T-VEC is the first prominent example.
T-VEC
Imlygic (talimogene laherparepvec, or T-VEC) is a local immunotherapy treatment that kills melanoma cells in the skin and lymph nodes and also produces an immune response by releasing antigens shed from the tumor cells and a protein (GM-CSF) that stimulates the immune system.
The final analysis of the OPTiM study (a randomized Phase 3 clinical trial to evaluate the efficacy and safety of treatment with T-VEC compared to subcutaneously administered GM-CSF in Melanoma patients with unresectable Stage IIIb, IIIc, and IV disease), at 49 months median follow-up, the durable response rate (DRR) increased from 16 to 19% compared to 1.4% in the GM-CSF arm; and the objective response rate (ORR) increased from 26% to 31.5% vs 6.4%. The results showed that T-VEC results in delayed response, as has also been seen with Ipilimumab (Yervoy) and other immune checkpoint inhibitors (ICI).
ATTACKING THE STING PATHWAY
A new breakthrough in melanoma models discovered that one of the reasons T-cells are found in melanoma is due to STING: The cGAS–STING pathway can detect cytosolic DNA and induce an immune response from it. Upon binding DNA, the protein cyclic GMP-AMP Synthase (cGAS) triggers a reaction of GTP and ATP to form cyclic GMP-AMP (cGAMP). cGAMP binds to Stimulator of Interferon Genes (STING) which triggers phosphorylation of IRF3 via TBK1.
Oncolytic viruses can activate the cGAS-STING pathway and utilize Akt1 kinase to block STING activation. Because of the abnormal DNA accumulating in melanoma cells, the hypothesis is that it will trigger STING, which will then begin an innate immune response, which leads to chemokine release and then the recruitment of lymphocytes.
Scientists then hypothesized if STING might also be playing a role with T-VEC? They discovered that many of the DNA viruses are known to phosphorylate AKT1 (one of 3 closely related serine/threonine-protein kinases, alongside AKT2 and AKT3), which can then inhibit a cGAS, blocking the STING pathway, allowing the virus to continue to replicate.
STING knockdown by shRNA (short-hairpin RNA: an artificial RNA molecule with a tight hairpin turn that can be used to silence target gene expression via RNA interference) demonstrated how we could reintroduce T-VEC’s oncolytic capabilities by knocking out STING in the cell lines.
Even with unreceptive tumor cell lines like DM4 (a spontaneous melanoma), modified T-VECs show activity ahead of PD-1 inhibitors and an improvement in overall survival.
Adding treatment to the T-VEC mix
Are there improvements to T-VEC? Studies have recently started on agents that are known to affect intracellular pathways. In melanoma, the MAP-kinase pathway is a good starting point, as 6 approved treatments targeting BRAF and MEK.
Though vemurafenib (targeting BRAF) showed no added benefit, trametinib (targeting MEK) showed that we could improve the viral replicative abilities and increase the lysis of the cells.
This was then tested in vivo, using a human xenograft model (the SKMEL-28 line). While both monotherapies (T-VEC and trametinib) actually decreased the tumor growth rate, the combination led to complete regression of tumors. As you can see below, the Ki67 staining showed there were fewer proliferating cells, demonstrating the increase in viral accumulation that occurs with the combination, compared to T-VEC alone.
Also, looking at apoptosis, there was an increase in apoptotic capabilities with the combination. We see below, the decrease in phosphorylation of Perk 1 / 2 and an increase in cleaved caspase-3
Studies have also been done on the antigen specificity of these T-cells. They found that if you treat with T-VEC, there is an increase in HSV-1 glycoprotein B specific T-cells, which are not seen in the MEK-inhibitor alone group, which is further increased with the combination, suggesting that we are getting much stronger anti-viral immune responses. Also, in 2 other melanoma differentiation antigens: gp100 and TRP-2, the quantity of these T-cells was significantly increased with combination therapy.
Gene expression analysis was also conducted, looking at the 18 pro-inflammatory interferon-gamma gene signature panel, that can predict responses to pembrolizumab therapy and predicting responses to treatment. It showed, that with the combination, almost all of the genes associated with interferon-gamma inflammation were greatly increased.
The biggest threat to oncolytic virus therapy is that if you have a good type 1 interferon response, you will upregulate PD1 and PD-L1 and this should shut down the T-cells. As you can see below, both PD-1 and PD-L1 are significantly increased by the combination. This suggests that combining this with the PD-1/PD-L1 blockade would be very effective.
Indeed, in animal studies, adding a PD-1 blockade led to a 90% survival compared to 60% with the PD-1 blockade alone. This 3-drug regimen also led to a further bump in the CD-8 T-cells, which suggests we can further drive the immune response.
T-VEC with Ipilimumab
In a study of T-VEC with ipilimumab vs ipilimumab alone, the combination was associated with a 39% objective response rate vs 18%, with visceral lesion response rate being 35% in the combination compared to 14% with ipilimumab alone. There were also no additional toxicities compared to ipilimumab alone.
T-Vec with Pembrolizumab
In a much smaller study, a response rate of 62% was seen with T-VEC and pembrolizumab, with about 33% having complete responses.
Though more studies need to be done, data shows how the oncolytic virus is able to take cold, unresponsive tumors and make them hot. While this preclinical data is impressive and clinical data for oncolytic adenoviruses have shown efficacy, more powerful treatment strategies are needed to achieve long-term tumor control in patients. As further understanding of individual patient-tumor immune status occurs, we will be able to treat advanced cancer patients better. It appears that oncolytic viruses will be a potent part of the future in multimodality approaches.