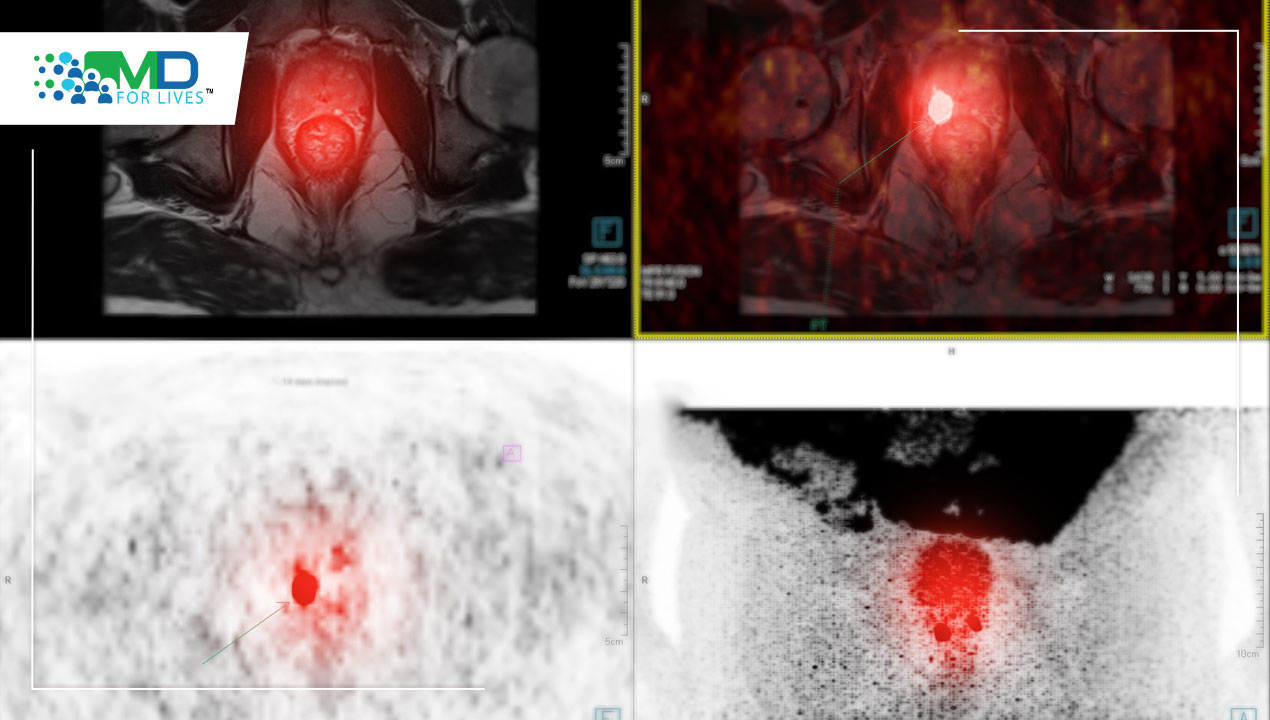
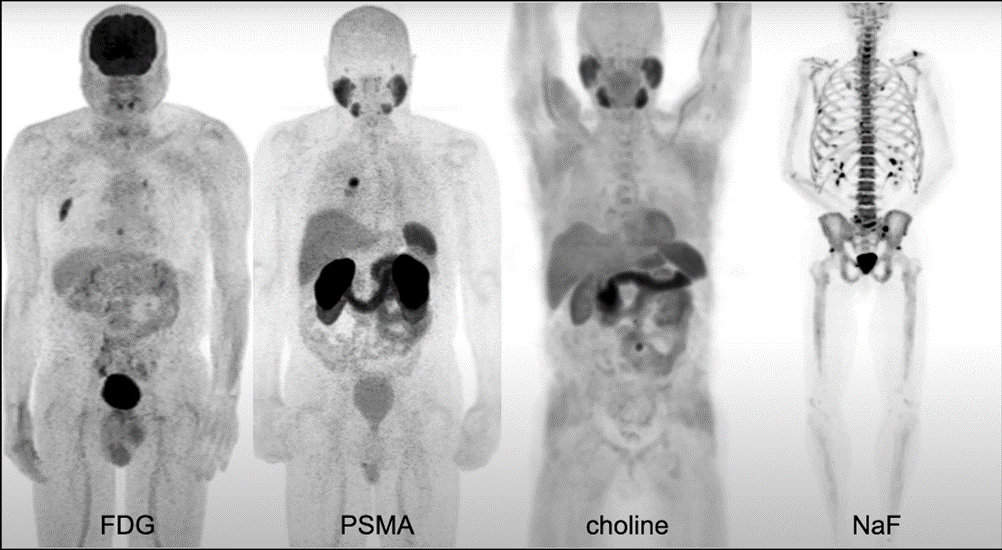
There are a number of radiotracers used in prostate cancer.
You have:
- Fluorodeoxyglucose (FDG), a sugar analogue (sometimes called a sugar PET) that can be used particularly in patients with the later-stage disease and can be prognostic but is not very helpful in localizing small volume disease.
- Choline is used frequently in prostate cancer
- Sodium Fluoride F 18 (18F-NaF), which can be used to visualize osseous metastatic disease.
- Prostate-specific membrane antigen (PSMA) is the newest generation agent.
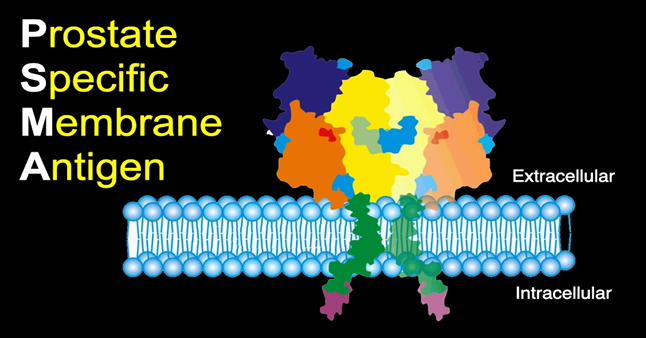
PSMA is a protein that lives on the membrane of prostate cancer cells and is overexpressed in prostate cancer compared to other tissues in the body. By targeting this protein, we can see where proteins are in the prostate cancer membrane and can localize prostate cancer metastasis fairly accurately.
There are a number of PSMA-radiotracers in use or being developed, their molecular structures you can see below. They each have very similar specificity and sensitivity for detecting prostate cancers
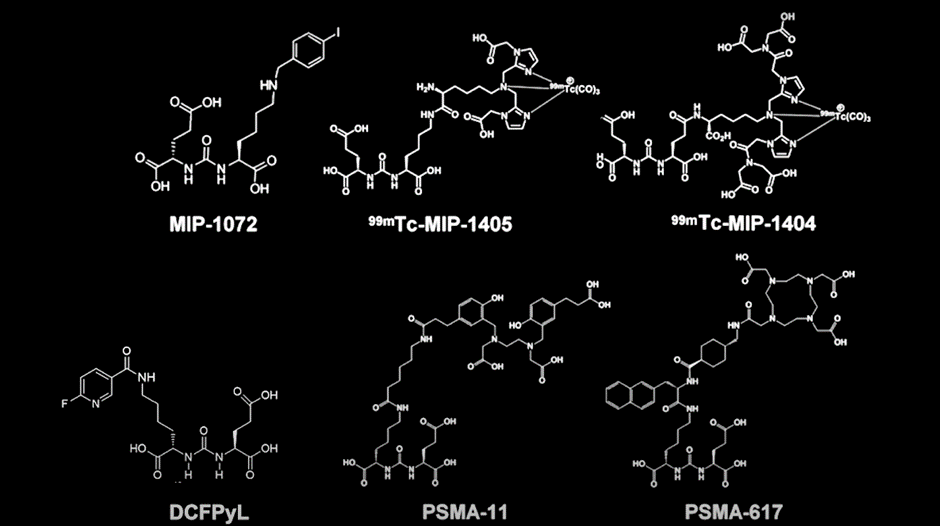
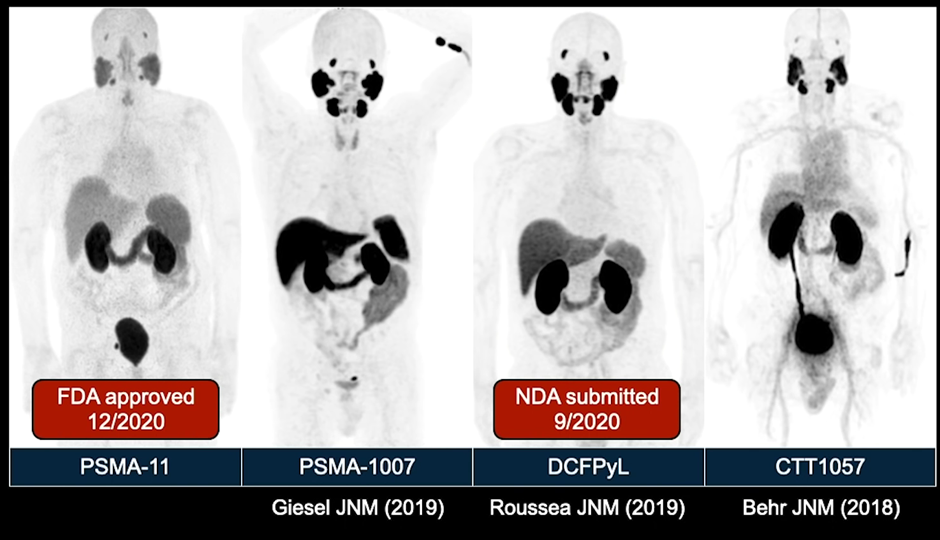
Their differences are based on biodistribution. As you can see below, PSMA-11 and DCFP-Yl are similar. PSMA-1007 (currently in phase 3 clinical trials), however, has a different biodistribution, with much more activity in the liver and less activity in the urine. In head-to-head studies, no radiotracer was shown to be better than the other and they all function nearly identically.
A popular PSMA molecule is gallium-68-PSMA-11. This is a radioactive diagnostic agent indicated for PET of PSMA-positive lesions in men with prostate cancer. Specifically, those with suspected metastasis who are candidates for initial definitive therapy and those with suspected recurrence based on elevated serum prostate-specific antigen (PSA) levels (this is in distinction to other drugs, such as fluciclovine or choline, which are only FDA approved in the setting of biochemical recurrence.
In December 2020, 68Ga-PSMA-11 was FDA-approved.
You can see its molecular structure below:
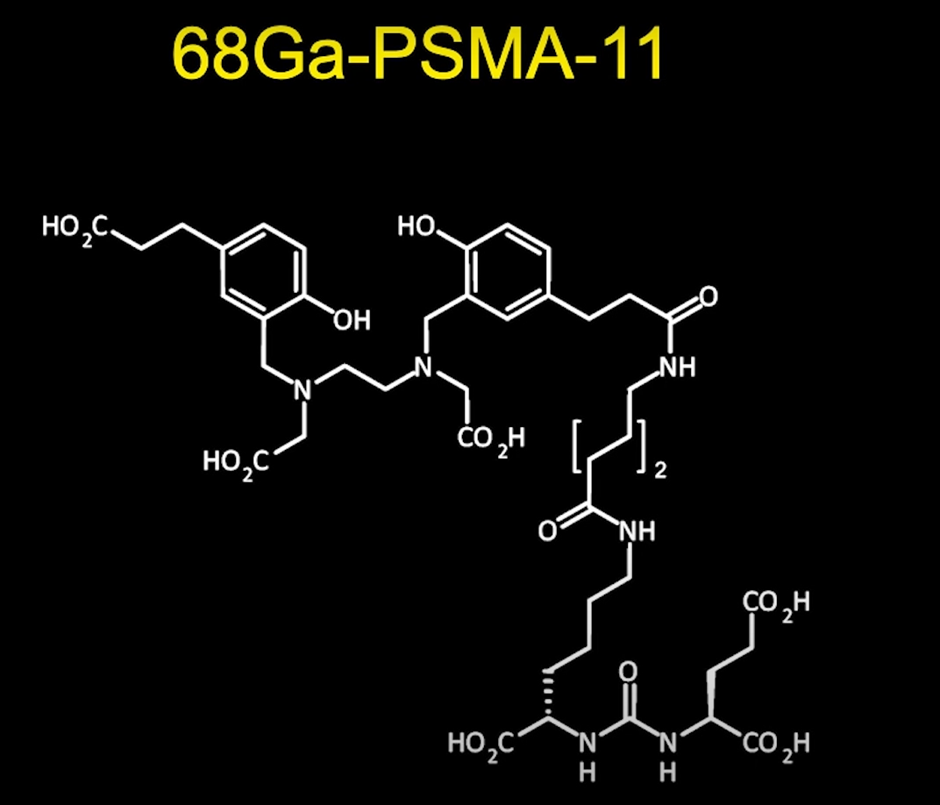
We then put Gallium-68 into this molecule and inject it into patients.
PSMA is injected into the arm of a patient.
The molecule then circulates throughout the body of the patient and binds to the PSMA molecule on the surface of tumor cells, and once it gets within the tumor cells, the gallium will then decay, giving off radioactivity that can be imaged using a PET (positron emission tomography) scanner.
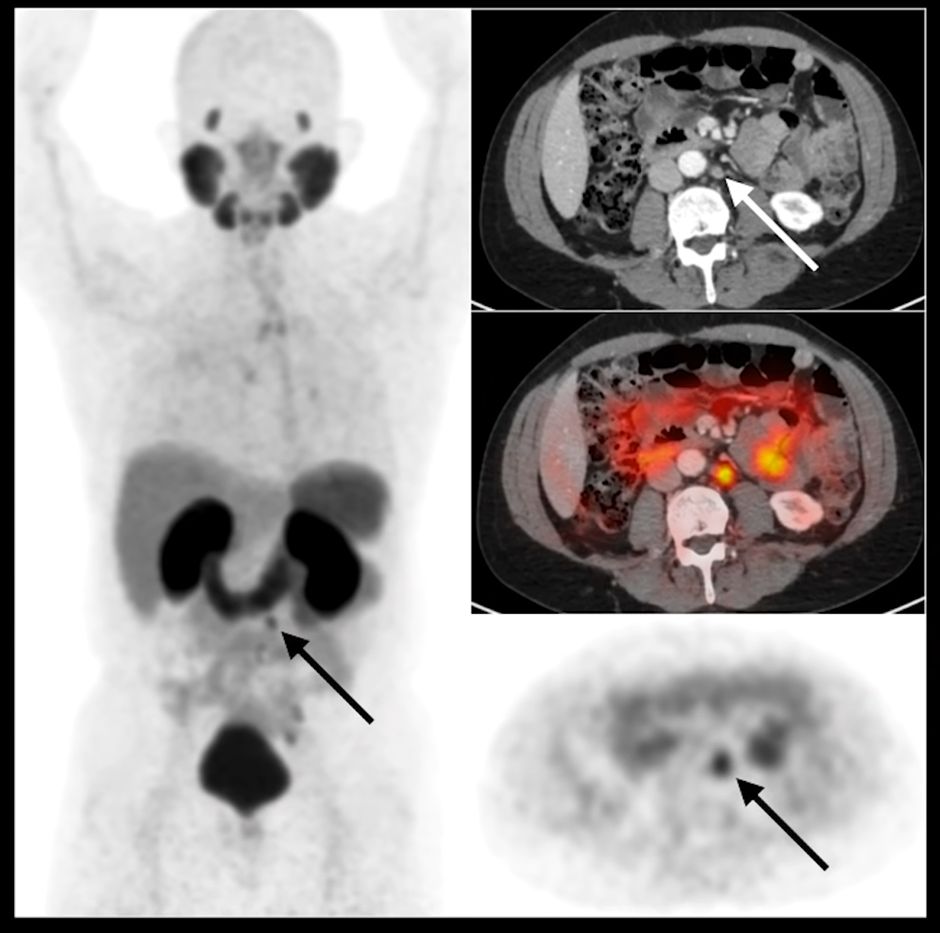
Without the PSMA PET to show that there is uptake consistent with metastatic prostate cancer, it would be difficult to determine if this was a metastatic disease, and this is where PSMA PET shines.
In a year or 2, there is a likelihood that radiology clinics will get to a place where staging with prostate cancer will be done primarily with PSMA PET, and no longer will patients be getting a bone scan, a CT scan, or an MRI. There will be a single imaging study that will be able to stage the entirety of the patient (both bone and soft-tissue staging).
How is PSMA compared to Fluciclovine?
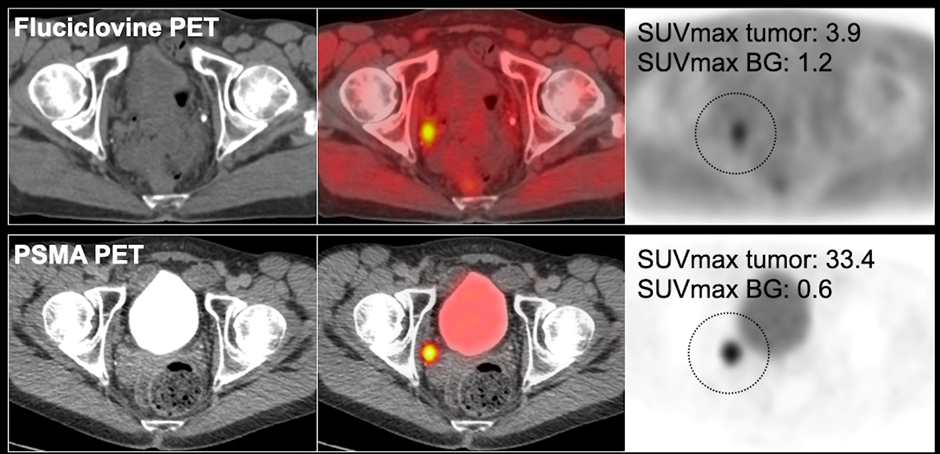
Fluciclovine is widely available in the US, is reimbursed by Medicare, and is currently the standard imaging modality for prostate cancer patients. Moving forward, we anticipate that PSMA will replace fluciclovine. Below, on the fluciclovine PET (top row), you can see that there is focal uptake in the right pelvic sidewall lymph node, which you can also see on the PSMA PET.
You can see that the uptake on the tumor in the fluciclovine is 3.9, compared to the adjacent background, which is about 1.2 (a 3 to 1 ratio between the tumor to background).
In the PSMA PET, the tumor background ratio is more than 30, and that makes it much easier to localize and see the disease, i.e., how much more the ratio that goes into the tumor and how much less there is in the background. This contrast allows radiologists and nuclear medicine physicians to localize the disease. In the case study below, both of the imaging studies localize the disease.
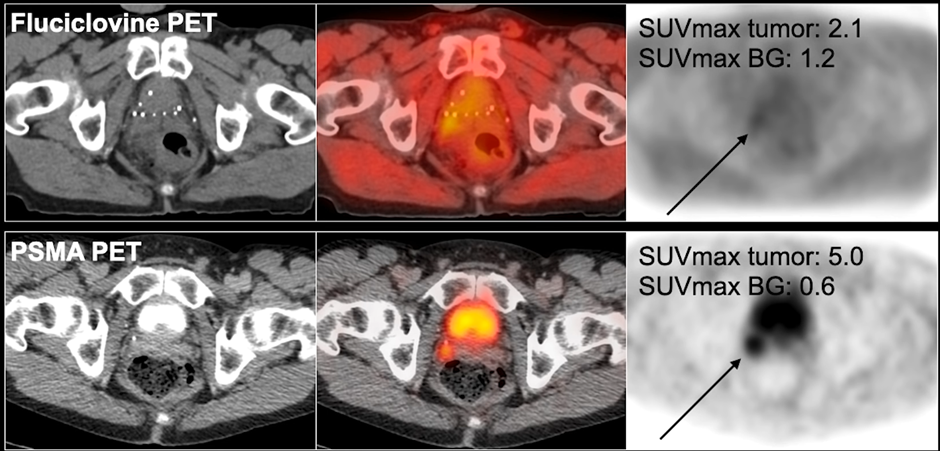
In the study below, in a different patient, you can see in the right seminal vesicle, there is a very subtle uptake in the fluciclovine PET (though there is a higher uptake in the tumor compared to tumor – about 2 to 1 ratio).
The PSMA PET, however, has a much higher focal uptake, which is much easier to visualize. In spite of the fact that there is also activity in the bladder at the same time, you can clearly see this local recurrence, considerably better than fluciclovine PET.
UCLA conducted a head-to-head study between PSMA and Fluciclovine, which is referred to as FACBC in the chart (Calias, Lancet Oncology 2019), and discovered that fluciclovine had half the detection rate compared to PSMA, which detected disease and metastasis at a much higher rate:
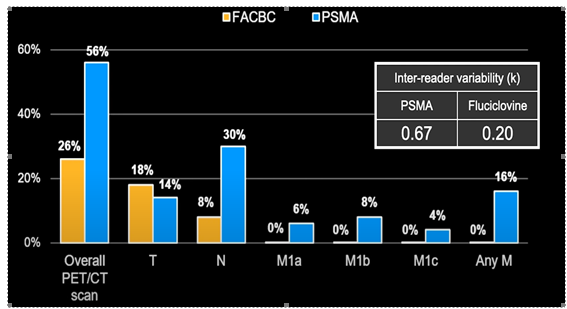
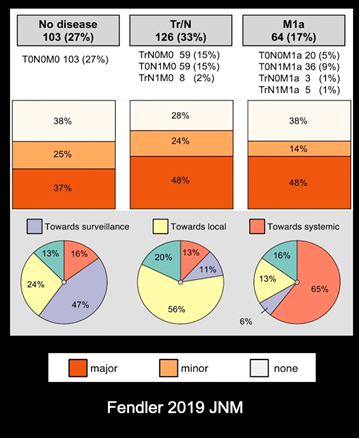
PSMA-PET results have a significant impact on disease management. When considering the location of a disease, patients may have:
- Negative PSMA-PET, so they are put on surveillance.
- Nodal disease (nodes in the pelvis or prostate bed), so a patient is treated with local therapy or radiotherapy to the nodes, or oligometastatic disease that was visualized.
- Metastatic disease – Instead of targeted treatments or active surveillance, these patients’ management has shifted to systemic therapy.
What about biochemical recurrence after radical prostatectomy?
A study by Boreta, in Urology magazine 2019, looked at 125 patients with PSA <2.0 after radical prostatectomy. 53% of those patients had PSMA+ disease, and more interestingly, of those 125 patients, 30% of them had their disease missed by standard radiation therapy fields (shown in green in the scans below). They are disease conditions that would not have been treated unless PSMA was done.
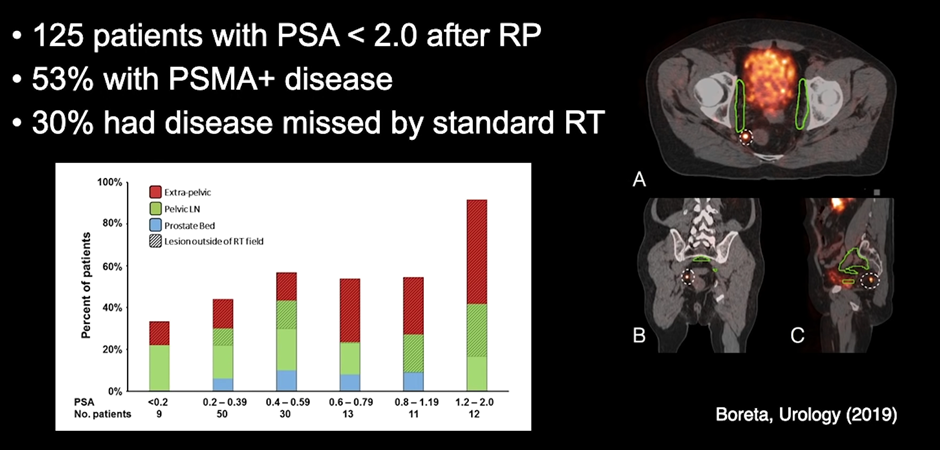
Radiotherapy techniques work well, providing the radiologist knows where to locate the tumor, with PSMA, that margin of doubt reduces, and molecular imaging like this, guiding radiotherapy can substantially improve patient outcomes.

MDForLives is a vibrant community of healthcare professionals and patients dedicated to shaping the future of healthcare. We provide valuable global insights to healthcare companies through online surveys, interviews, and discussion forums.