August is Psoriasis Action Month, an event created by the National Psoriasis Foundation to educate the public and raise awareness. In honor of this occasion, let’s talk about some recent developments in psoriasis research and treatment.
Psoriasis causes and manifestations
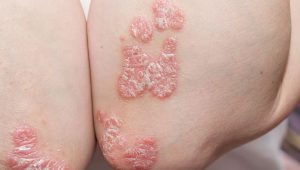
Psoriasis is much more than a rash. It is a chronic, systemic inflammatory condition that affects as many as 8 million people in the US alone. This inflammation commonly manifests as a rash, with areas of skin in which keratinocytes multiply much faster than normal. Several subtypes of psoriasis rashes exist, with different appearances, and can affect the limbs, scalp, trunk, nails, or even the entire body.
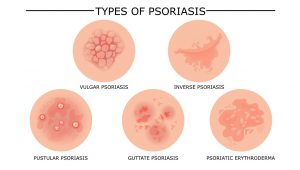
In up to 30% of patients, psoriasis can also affect the joints (psoriatic arthritis) and can cause permanent joint damage if it is not effectively treated. In about 10% of patients, psoriasis causes ocular symptoms or complications, including uveitis, and can affect vision. People with psoriasis may also be at greater risk for other health problems, including inflammatory bowel diseases, heart disease, certain cancers, and depression.
Drugs including corticosteroids, interleukin inhibitors, methotrexate, and others are used to treat psoriasis. Older psoriasis treatments include coal tar and phototherapy, as well as exposure to sunlight or seawater.
What is Psoriasis Action Month?
The National Psoriasis Foundation, a US organization, founded Psoriasis Awareness Month in 1997. In 2017, to celebrate the growing availability of psoriasis treatments and the advancements in the understanding of the disease, the name was changed to Psoriasis Action Month.
Every August, Psoriasis Action Month is an opportunity to increase public understanding of psoriasis, reduce misconceptions and stigma, and a chance for people with psoriasis to tell their stories.
Long-term data on a pediatric psoriasis treatment
A recent clinical trial publication showed that a monoclonal antibody, ixekizumab, can control children’s psoriasis symptoms long term. Ixekizumab (sold as Taltz) is an inhibitor of interleukin 17A.
In 2020, the FDA approved ixekizumab as a psoriasis treatment for use in children ages 6 and up. The approval was based on initial results from the IXORA-PEDS study. Long-term results from the same study were published in JAMA Dermatology on April 13, 2022.
Children between the ages of 6 and 18 who had moderate to severe psoriasis were randomized in a 2:1 ratio to receive ixekizumab (115 patients) or placebo (56 patients) for 12 weeks. Then, all patients could receive ixekizumab every 4 weeks during an open-label maintenance period, followed by an extension period to week 108. Patients treated with ixekizumab were also compared to a reference group treated with another therapy, etanercept.
Efficacy and safety results were consistent with previous shorter-term data. Through week 108, 91.7% of patients achieved 75% improvement from baseline in the Psoriasis Area and Severity Index, and 55.1% achieved 100% improvement from baseline. During a randomized withdrawal period, 90.9% of patients receiving a placebo relapsed, compared with 17.6% of patients on an active drug.
Observed adverse events were consistent with the safety profile of ixekizumab and included treatment-emergent infections in 74% of participants; allergic reactions, cytopenia, hepatic issues, and depression were observed in several patients, as well as one patient with a malignant neoplasm.
Air pollution may trigger psoriasis flare-ups
For many patients, psoriasis flare-ups, or exacerbations, occur after the patient experiences triggers, which can include weather conditions, skin injury, stress, infections, and the use of certain medications.
A recent study in Italy found an association between air pollution severity and psoriasis flare-ups. The study used a case-crossover design, in which each participant (with existing psoriasis) serves as his or her own control. The researchers found that concentrations of air pollutants were significantly higher in the 60 days preceding a psoriasis exacerbation compared with the 60 days preceding a control visit.

Psoriasis may increase the risk of ectopic pregnancy
A third study, this one investigating adverse pregnancy outcomes, found that women with psoriasis may be at increased risk for ectopic pregnancy. This case-control study was conducted in Denmark using data from the Danish National Patient Registry. The researchers compared women with an adverse pregnancy outcome (N=42,041) and women with a live birth (N=449,233), using psoriasis as an exposure. Women with psoriasis were 1.31% of the total.
The analysis suggests that ectopic pregnancy is more frequent among women with psoriasis (1.87%) than those without (1.5%), with a crude odds ratio of 1.25 (95% CI, 1.06-1.50). The risks for other adverse pregnancy outcomes were not increased.
Psoriasis is a difficult disease, but newer treatments and our growing understanding of the underlying biology are improving the lives of patients today and providing hope for the future.

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.