Glaucoma is a group of eye illnesses that impair the optic nerve, which is necessary for vision. Glaucoma commonly leads to blindness.
Untreated glaucoma can hasten the progression of permanent vision loss or blindness. The diagnosis and treatment of glaucoma are far more complex than those of cataract and necessitate a much higher level of clinical expertise. The difficulties in managing glaucoma are numerous. However, with new devices recently entering the market and others in the final stages of development, specialists’ options for intervening across the spectrum of glaucoma severity and subtype with interventions that do not rely on patients’ compliance are expanding. Because of their potential to save or reduce medication use and improve quality of life, procedural options force clinicians to reconsider what constitutes a successful outcome.
Fluid in the eye does not drain properly in people with high-pressure glaucoma, causing pressure on the optic nerve and vision loss. While there are a few treatments for open angle glaucoma, the most common form of glaucoma in adults (eye drops, oral medicine, laser treatments), there are no cures, and primary congenital glaucoma, a severe form of glaucoma in children from birth to three years, can only be treated with surgery.
New medicines and therapy classes are desperately needed to reduce both types of visual loss. Gene editing was employed to develop new glaucoma models in mice that were equivalent to primary congenital glaucoma. The scientists were able to replace the function of genes that cause glaucoma by injecting a new, long-lasting, and non-toxic protein therapy (Hepta-ANGPT1) into mice. The scientists were also able to prevent glaucoma from developing in one model with this injectable medication. When injected into the eyes of healthy adult mice, the same medication lowered eye pressure, indicating that it could be a new class of therapy.
For decades, patients with mild to moderate illness who had uncontrolled intraocular pressure (IOP) despite medication and laser treatments left the doctors with a treatment gap with limited options.
However, in the last two decades, minimally invasive glaucoma surgery (MIGS) has emerged to satisfy the unmet need for better treatment choices and close the treatment gap. MIGS reduces IOP by focusing on different components of normal aqueous dynamics. The first method involves increasing outflow through Schlemm’s canal and the trabecular meshwork. The juxtacanalicular trabecular meshwork has long been thought to be the site of maximum aqueous outflow resistance. This resistance can be circumvented by bypassing or eliminating this tissue and increasing outflow to lower IOP. A trabecular meshwork bypass stent, which allows water to flow straight from the anterior chamber into Schlemm’s canal, can be used to achieve bypass.
Goniotomy or trabeculotomy, which involves a surgical incision and/or excision of this tissue and allows for enhanced aqueous outflow into Schlemm’s canal, is another way to bypass the trabecular meshwork’s barrier. Another way to promote outflow through the normal physiologic aqueous outflow system is to dilate Schlemm’s canal with cannulation and expand it with viscoelastic.
In 2012, the FDA approved the iStent Trabecular Micro-Bypass Stent (Glaukos, Laguna Hills, CA) as the first MIGS implant. A heparin-coated, non-ferromagnetic titanium stent was used. The device has a height of 0.3mm and a length of 1mm. A snorkel with a central lumen of 120 microns and a height of 0.25mm protrudes into the anterior chamber. Three retention arches are included in the body of the device that is implanted into Schlemm’s canal to guarantee that it stays in place. The iStent is preloaded into a sterile injector that can only be used once.
The iStent’s pivotal experiment compared the efficacy of phacoemulsification alone against phacoemulsification with iStent insertion. Eyes (240) with mild to moderate glaucoma and intraocular pressure less than or equal to 24 mmHg were included in the prospective, randomized experiment. Patients were randomly assigned to receive either phacoemulsification alone or phacoemulsification with iStent insertion: 111 patients got iStent placement during phacoemulsification. Patients were tracked for a year, with the primary endpoint being an IOP of less than 21 mmHg without the use of ocular hypotensive drugs. At 12 months, 72 percent of eyes treated with an iStent and phacoemulsification had achieved the primary objective, compared to 50 percent of eyes treated with phacoemulsification alone. Furthermore, 66 percent of iStent eyes experienced an IOP drop of greater than or equal to 20%, compared to only 48 percent of control (phacoemulsification only) eyes. At one year, both groups experienced statistically significant reductions in intraocular pressure, and there was no difference in adverse effects.
Ivantis Inc. created the Hydrus Microstent, which was approved by the FDA in August 2018. The microstent has three windows and an intake in the anterior chamber and is 8mm long and 290 microns in diameter. It is made of nitinol, a biocompatible titanium and nickel alloy that has previously been employed in cardiac stents. At a 90-degree angle, the device crosses the trabecular meshwork. A trabecular meshwork bypass stent and a Schlemm’s canal patency scaffold are used to lower intraocular pressure. The HORIZON trial, which compared phacoemulsification alone to phacoemulsification with Hydrus microstent implantation, was essential in the Hydrus microstent’s approval. The Hydrus group also had considerably lower use of hypotensive eye drops after 24 months than the phacoemulsification alone groups.
Trabecular meshwork bypass can also be performed with surgical incision/excision of the trabecular meshwork tissue, in addition to the stents discussed above. The capacity to circumvent the trabecular meshwork and provide a direct pathway to Schlemm’s canal for 3-4 or more clock hours is one of the main benefits of MIGS operations. Importantly, there is no need for an implant, and these goniotomy/trabeculotomy operations can be performed alone or in conjunction with cataract surgery.
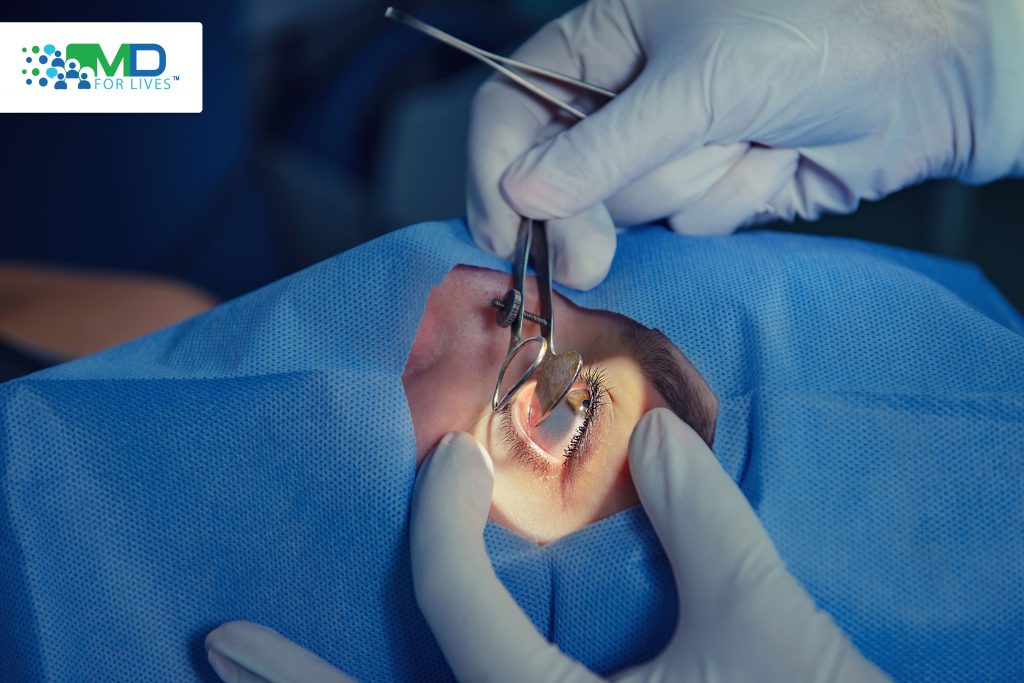
Considering all the advantages and coexisting other morbidities of the old age, the advent of minimally invasive glaucoma surgery has opened a new chapter in the armamentarium of glaucoma management. iStent and Hydrus devices are gaining popularity among glaucoma specialists and cataract surgery for their ease of use during cataract surgery.
Knowing the history of MIGS, the pivotal clinical trials of some of the most commonly used devices such as Hydrus, iStent before exploring the cost-benefit and quality of life for the patients within the field, and real-world data to sum-up the evidence for these techniques is important for physicians to save, extend, and improve patients’ lives.
Dr. Pouya Alaghband, a Consultant Ophthalmic Surgeon, has vast knowledge in the field of ophthalmology who applies and devotes his expertise to identifying the patient’s medical problem and then using his abilities to prevent or treat it. He has a strong interest in glaucoma and finished his MD(res) on aqueous humour dynamics using MIGS devices at King’s College London. He has participated in groundbreaking research such as EAGLE and PTVT, as well as commercial trials such as ATHENA and TRITON. He is the primary investigator for the TARGET and CONCEPT studies at the moment. He has published extensively on the subject of glaucoma and has given talks both nationally and internationally.
In the upcoming webinar organized by MDforLives on May 19th at 7 PM GMT exclusively for physicians, Dr. Alaghband will talk about MIGS’ history, as well as critical clinical trials for some of the most regularly used devices like Hydrus, iStent, before delving into the cost-benefit and quality of life for patients in the field. Finally, real-world data will be presented to summarize the evidence for these strategies before recommending future directions for some of the field’s ongoing clinical trials. At the conclusion of the meeting, there would be an opportunity for questions and answers.
Registration is just pen for a few more days and seats are very limited. So, do not delay to book your seat and increase your expertise in treating glaucoma.