The esophago-gastric junction (OGJ) is located between the esophagus and the stomach.
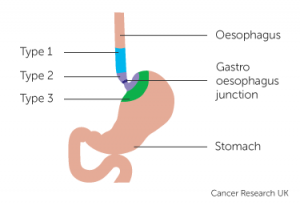
Adenocarcinomas of the OG junction have been increasing steadily over the last c50 years:
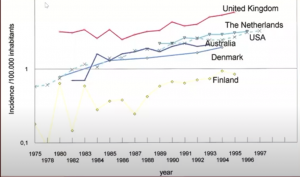
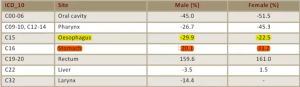
There has been a 20-30% reduction in esophageal cancers, but also a 20-30% increase in stomach cancers, with the majority of these being OG junction adenocarcinomas, a rate particularly prevalent in males, as seen in the incidence chart below:
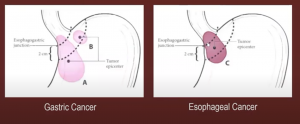
The OG junction is seen as ‘in no man’s land’, it is partly intra-thoracic and partly intra-abdominal. What is gastric cancer and what is esophageal cancer?
There is also a mix-up in diagnosis between adenocarcinoma and squamous cell carcinoma, based on margins, nodal dissection (should it be confined to the abdomen or also the thorax), the surgical approach (trans-abdominally vs trans-thoracic vs trans-hiatal?), and what is the right approach in terms of multimodality (chemotherapy, radiotherapy, pre-operative, post-operative?). All of these questions have affected not only diagnosis and treatment paradigms, but also affected guideline organizations, giving the best recommendations to pathways of management.
Etiology: Factors prevalent in OG in carcinoma include alcohol and tobacco history, diet and nutrition, HPV, physical activity, as well as H.pylori, GERD disease, obesity, and Barrett’s syndrome.
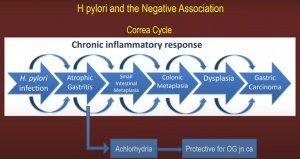
A recent study argued that H.pylori eradication treatment did not lead to an increase in OG junction adenocarcinoma according to a study by Doorakkers et.al (Wiley 2020).
Barrett’s esophagus however is a positive prognostic factor indicating OG Jn adenocarcinoma.
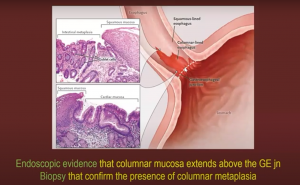
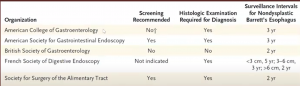
Though different societies still have differing guidelines on this:
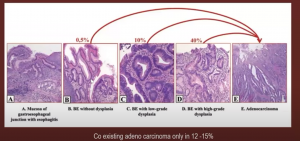
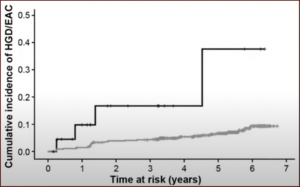
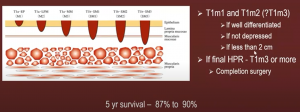
What of progression to malignancy? You can see below the risk increases from 0.5% for BE without dysplasia, to 40% in patients with Barrett’s esophagus high-grade dysplasia.
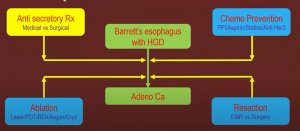
How do you control symptoms and the risk of progression? Options vary between radiotherapy, chemotherapy, ablation, and resection.
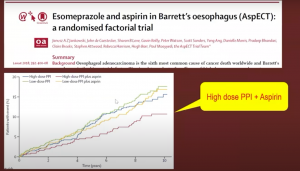
In anti-reflux therapy, there is nothing to choose between surgical approach vs medical measures (aspirin + high dose proton pump inhibitor), the outcomes are similar in terms of symptom control, though if you want to lower the incidence of malignancy, medical measures (black line in the graph below) have shown better outcomes:
In the AspECT trial (Lancet 2018), you can see this medical measure benefit:
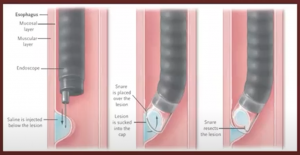
Resection: In terms of prevention of malignancy, resections are preferred over ablations, as it gives histological confirmation, the margins are easily assessed, and further radiotherapy can be planned.
A typical flow chart for Barrett’s disease can be seen below:
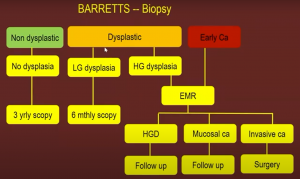
If the tumor is confined to T1m1 and T1m2, a submucosal resection is recommended.
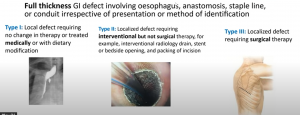
Anastomotic leaks: These significantly increase morbidity and mortality, affect a patient’s quality of life, also expensive to the health service, and lead to early disease recurrence and poor survival; you can see the types below:
Investigations:
A CT- chest and abdomen is recommended, as is an EUS ultrasound, which is superior to CT in T1/2/periesophageal nodes/FNAC identification, though not as useful in T3/4 lesions and obstruction lesions, plus sensitivity and specificity are lesser at OG Jn. A PET CT is recommended in locally advanced lesions, these upstage 15% of patients and change treatment plan in 20%. Laparoscopy is very useful in recommending patients change to radiotherapy or avoid laparotomy.
Treatment plan for SCC esophagus:
Total esophagectomy, stomach tube, neck anastomosis, McKeown (thoracic/abdominal/cervical parts), and trans hiatal approaches are all options based on margins:
Below you can see the margins in OG Jn tumors:

Below you can see a cervical anastomotic leak Type 1:
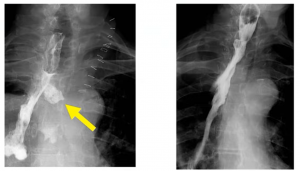
A radiological intra-thoracic Type 1 leak:
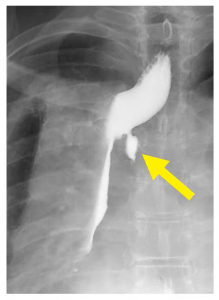
A Type 2 leak can be very large:
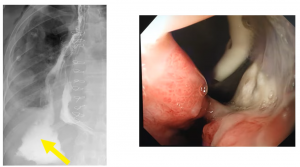
Management of ADC – Type II: Total gastrectomy and esophagojejunal anastomosis are recommended.
Surgical management is summarized below:

Patient factors to consider:
Patients should stop smoking, should have aggressive chest physiotherapy (continued throughout the NAC period), and should also have nutritional rehabilitation. In terms of intraoperative discipline, ensure: single lung/double lung ventilation; a minimally invasive approach should be considered; standardize the step-by-step approach (i.e., creation of the stomach tube); avoid post-op ventilation; early ambulation/physiotherapy should be considered; enteral nutrition may be necessary; and prepare for early tracheostomy.
Beware of intraoperative complications (especially thoracic duct injury).
Diagnostic criteria and management include triglycerides, lymphocyte count in chyle, early re-exploration, and conservative management (nothing by mouth and total parenteral nutrition, fat-free diet, octreotide).
A gastric conduit technique is good in terms of vascularity, smooth pull into the neck, and safe anastomosis. In anastomosis, a neck leak is easier to manage and drain than an intrathoracic leak, which should be avoided at any cost.
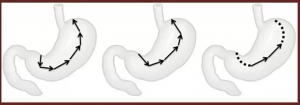
Neoadjuvant and adjuvant therapy in adenocarcinoma.
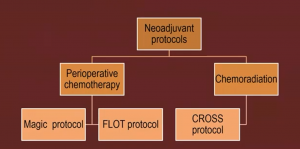
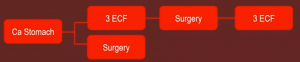
The MAGIC protocol can be seen below:
It was the first adequately powered random controlled trial, with good design and conduct; however, only 40% completed all chemo, with 26% OG Jn cases and 70% node positive.
FLOT trial: The FLOT (5Fu/Leucovorin/Oxaliplatin/Taxane) trial showed marked improvement compared to MAGIC
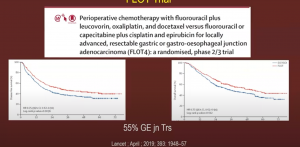
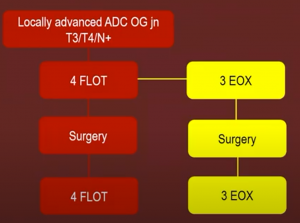
General consensus:
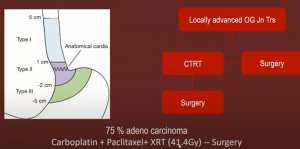
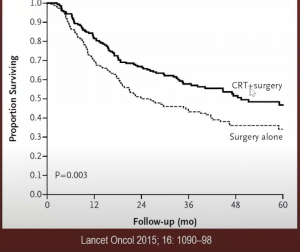
While in the proximal stomach – the CROSS trial recommended neoadjuvant radiation, with 75% adenocarcinoma showed a significant improvement if you incorporated surgery plus carboplatin + paclitaxel + XRT (41.4Gy) compared to surgery alone.
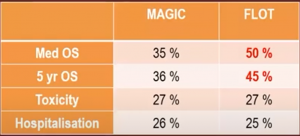
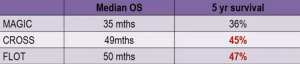
Similarly, FLOT showed better 5-year survival results compared to the MAGIC regimen:
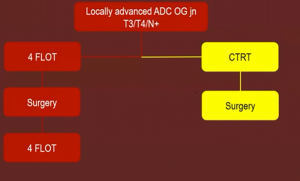
Issues with the CROSS protocol include the radiation field, lung toxicity, and desmoplasia in irradiated patients.
Results for TOP GEAR/CRITICS II/Neo-Aegis/ESOPEC trials are still being processed:

In esophageal and GEJ adenocarcinoma, neoadjuvant therapy is recommended with chemo RT as a standard alternative. FLOT is the new standard perioperative chemo of choice, though there are a number of comparative studies pending. In R0 resection, pCR, N0 status, and chemo and radiotherapy are recommended. While on the horizon, new agents from metastatic disease trials will soon be able to identify pathways, networks, and genomic analysis that may advance OG Junction care beyond the scalpel/radiotherapy/chemo standard of care, to a new realm of targeted drugs that could extend the life and be curative in the long run.

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.






