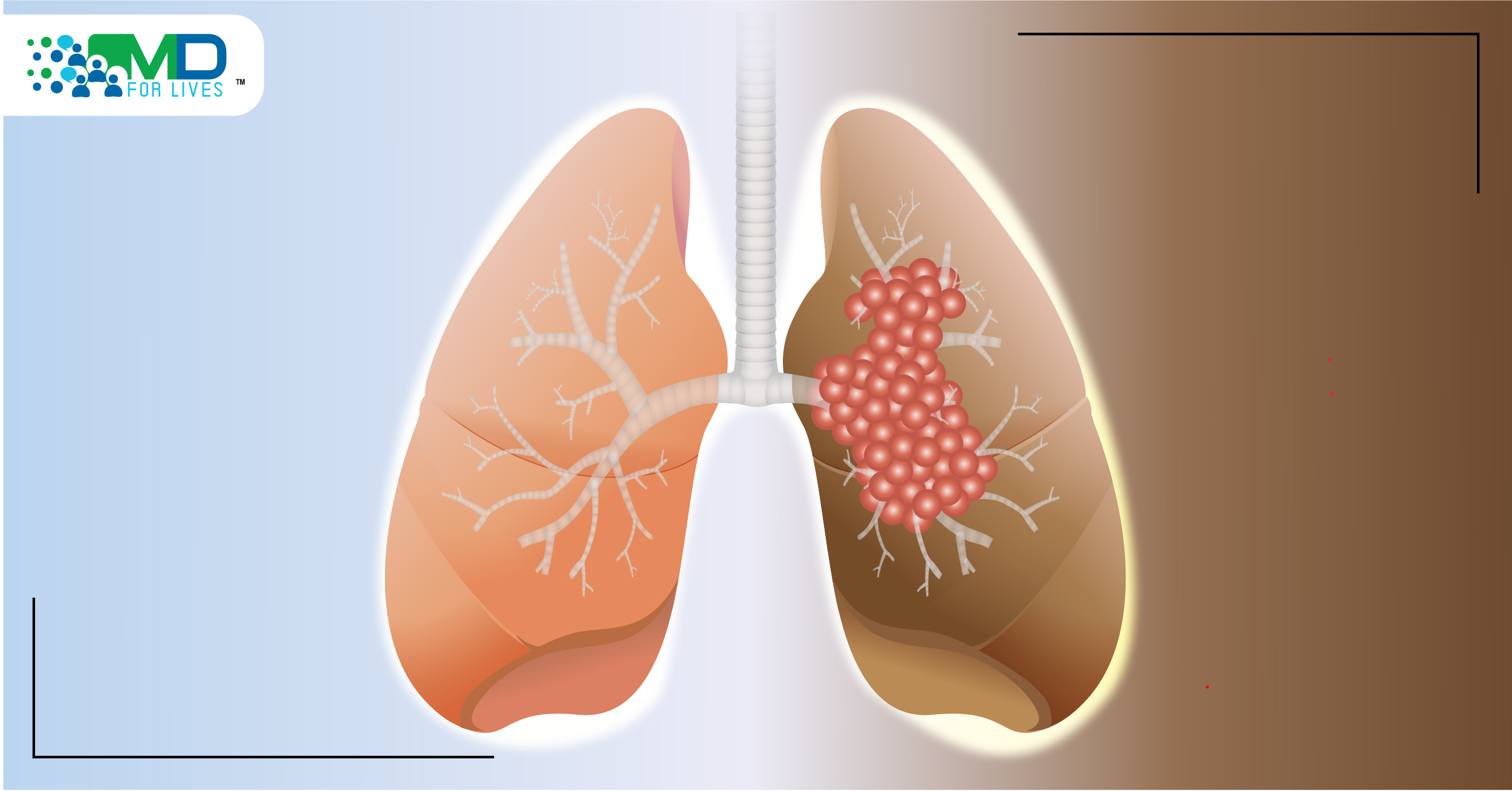
Lung cancer kills more people worldwide than breast, prostate, colorectal, and brain cancers combined.1 Non-small cell lung cancer (NSCLC) accounts for about 85% of the ~235,000 new cases of lung cancer per year in the US.1,2,3
Recent advancements in genetic analysis and lung cancer treatments that target specific genetic changes have helped reducing mortality and improve outcomes for patients with NSCLC.1,2,3 By 2014, incidence-based mortality among men who received NCSLC diagnoses had been reduced to 26% from 35% in 2001.1
New treatment targets for NSCLC
Many of the newer NSCLC drugs on the market target tumors with specific genetic changes. Accordingly, an increasing number of patients undergo testing to understand their lung cancer genetics before deciding on a treatment plan.1,2

Epidermal growth factor receptor (EGFR, ErbB) gene mutations are one of the most well-researched targets for lung cancer treatment.1,2 EGFR tyrosine kinase inhibitors (EGFR TKI) are used as first-line treatments in advanced NSCLC cases where EGFR mutations are present.1,2 The development of tumor resistance, however, is a major problem with this targeted therapy, which required the development of next-generation (up to 4th generation) drugs and the use of combination therapies.1,2
Many other genetic targets are under active research in lung cancer, including Kirsten rat sarcoma viral oncogene homolog (KRAS), c-mesenchymal-epithelial transition receptor (c-Met), anaplastic lymphoma kinase (ALK), c-ros oncogene 1 (ROS1), and neurotrophic tyrosine receptor kinase (NTRK).1 The human epidermal growth factor receptor 2 (HER2 or ErbB2) is a tyrosine kinase receptor that belongs to the EGFR (ErbB) family and is sometimes implicated in breast, lung, and other cancers.3
HER2-positive NSCLC targeted treatment
An estimated 2-6% of NSCLC cases have a mutation in the HER2 oncogene, and others have overexpression or amplification of HER2.3 Several HER2-targeted drugs are under study, though none has yet reached the clinic for NSCLC.3

Poziotinib is a drug that targets HER2 and several other ErbB receptors that are involved in various cancers; it is showing promise in clinical trials for NSCLC with a HER2 exon 20 mutation.4,5,6 The FDA gave fast-track designation to poziotinib in March 2021, which allows an earlier review and potentially earlier approval.5,6
Other examples are trastuzumab deruxtecan (T-Dxd) and ado-trastuzumab emtansine (T-DM1), which are HER2-targeted antibody-drug conjugates in trials for NSCLC that involves HER2 overexpression or mutation. Both have shown early success in Phase 2 trials in patients with advanced or relapsed disease, and T-Dxd received FDA Breakthrough Therapy designation in 2020.1,7,8,9

Other treatment targets for NSCLC
For other groups of patients with NSCLC, multiple drugs that have recently come on the market are in trials, depending on the genetics of their tumor type.1 In May 2021, the FDA approved the first KRAS mutation-targeting therapy, sotorasib, for advanced NSCLC with KRAS G12C mutations. This includes about 13% of patients with NSCLC.1,10
Amivantamab, which is a humanized antibody that targets both EGFR and MET, received accelerated FDA approval in May 2021 for the treatment of patients with advanced NSCLC with exon 20 insertion mutations.1,11
The kinase inhibitor capmatinib was approved in May of 2020 for metastatic NSCLC with mutations that lead to MET exon 14 skipping, which is found in 3-4% of lung cancer cases.1,12
Monoclonal antibodies that target other mutations in NSCLC, such as HER3, are currently in trials.1,2 Patritumab deruxtecan (HER3-DXd/U3-1402) is a HER3-targeted antibody-drug conjugate that showed anti-tumor activity and acceptable safety in patients with advanced NSCLC who are on EGFR TKI.1
Immunotherapies that involve immune checkpoint inhibition are also under investigation.2,13 Monoclonal antibodies against programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) are effective treatment options for a relatively small percentage of patients with NSCLC, but they are not recommended for patients with EGFR-mutated NSCLC.13 Treatment strategies that use immunotherapy in combination with other drugs are the subjects of several clinical trials.2,13
Research is ongoing into how best to use these new products for HER2-positive and HER2-negative NSCLC. Better strategies to overcome the tendency of NSCLC tumors to develop drug resistance will be the key.2 Other important factors include advances in lung cancer genetic analysis, the field’s thorough understanding of appropriate genetic targets, the ability to predict patients’ responses to targeted therapies, and strategies for avoiding unacceptable toxicity with combination therapies.1,2,3,13