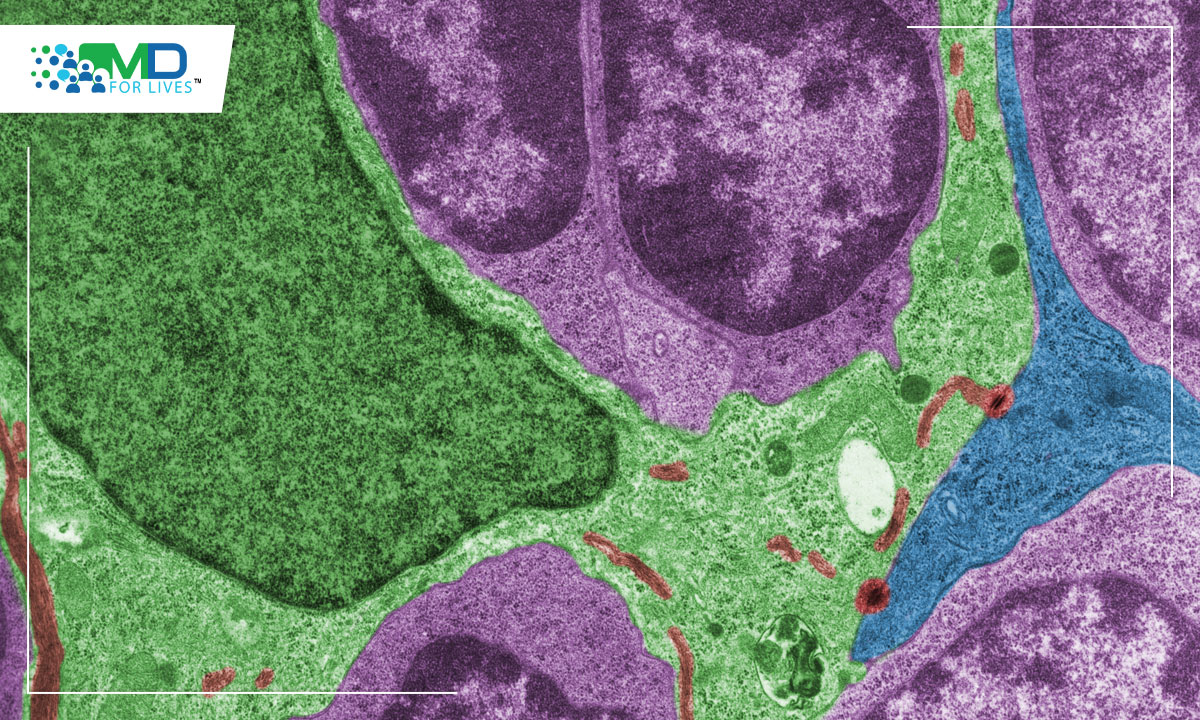
Scientists from the University of Missouri School of Medicine and College of Engineering described the thymocyte cells, an immune cell found in the thymus while investigating mice with T-ALL. They discovered that the same sort of T cell, which produces a particular collection of molecular markers, is the source of all mouse cancers. T-cell acute lymphoblastic leukaemia (T-ALL), which affects more than 6,000 Americans each year, may be caused by dysfunction involving a unique type of thymocyte cells that is present in very small numbers in every individual.
Everybody possesses a tiny number of unique thymocyte cells, and in some cases, these cells develop into leukaemia. T-cell acute lymphoblastic leukaemia (T-ALL) is an aggressive and rapidly progressing form of acute leukaemia. In contrast to acute lymphoblastic leukaemia (ALL), which often affects B lymphocytes, it affects stem cells that produce lymphoid cells, specifically a kind of white blood cell called T lymphocytes. One of three kinds of lymphocytes (white blood cells) develops from a lymphoid stem cell, which first becomes a lymphoblast cell. Antibodies are produced by B lymphocytes to aid in the fight against infection. T cells assist B lymphocytes in producing antibodies that aid in the defence against infection. They are natural killer cells that target viruses and cancerous cells. T-cell acute lymphoblastic leukaemia (T-ALL) accounts for roughly 12% to 15% of all newly diagnosed ALL cases in paediatric patients and is notable for its distinct clinical and molecular characteristics. Although T-ALL outcomes were previously inferior to B lymphoblastic leukaemia (B-ALL), with recent breakthroughs in therapy, event-free survival (EFS) rates have progressively improved and currently approach 85 percent in several modern clinical trials. Cure, on the other hand, has not come cheap, since rigorous therapy is necessary. Furthermore, recurring illness is extremely difficult to treat, and few novel medications for children with the resistant disease have been found. T-cell acute lymphoblastic leukaemia (T-ALL) is physiologically distinct from B-ALL and has distinct kinetic patterns of disease response. Although T-ALL and B-ALL are treated with relatively identical regimens, differences in responsiveness to key parts of treatment have been found.
After identifying the cell in mice, the researchers questioned if humans had the same cell type and amount. The results were in their favour, since the human samples they collected included identical T cells and in the exact quantity seen in mice. The name “EADN” was given to this unusual cell, which accounts for only 0.01 percent of all cells in the thymus gland. The scientists sought to determine if every human T-ALL incidence was caused by EADN. Over a three-year period, investigators at the University of Missouri Health Care analysed five T-ALL instances, examined cell samples from each patient, and concluded that one of the five cases appears to have started from an EADN cell. They were not claiming that EADN is the sole cell that produces this sort of cancer, but the data indicate that it is involved in certain cases. This is an interesting discovery, and they also discovered something distinctive about EADN cells. In mice, EADN cells are signalled to become cancerous by a protein called major histocompatibility complex (MHC), which triggers autoimmunity and other immune reactions. It’s similar to an auto-immune response that causes EADN to progress to cancer. Many other thymic cells are unable to do so. Now that they identified the signals essential for this transition, they may be able to develop treatment techniques.
The next step will be to identify how frequently human T-ALL cases are caused by EADN cells, with the goal of learning how to better customise therapy for each individual’s cancer case. Innovators from the University of Missouri and the three other research universities in the UM System come together as part of the NextGen Precision Health initiative to work on potentially game-changing advances in precision health. This initiative also emphasises the value of extensive interdisciplinary collaboration and the potential of personalised healthcare. It is a joint endeavour to use Mizzou’s research strengths to improve the health of Missourians and others. In the state-of-the-art research facility, the Roy Blunt NextGen Precision Health building at MU centres the broader programme and expands collaboration between academics, clinicians, and industry partners.
Reference: –
https://www.pnas.org/doi/full/10.1073/pnas.2118529119
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6142501/
https://www.leukaemiacare.org.uk/support-and-information/information-about-blood-cancer/blood-cancer-information/leukaemia/t-cell-acute-lymphoblastic-leukaemia/

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.