Patients with a genetic disease called alpha1 antitrypsin deficiency can develop both liver disease and lung disease, either of which can cause serious health complications or death. No pharmaceutical treatment for AAT deficiency-associated liver disease is yet available, and many patients eventually require a liver transplant.
In June, the authors of a small Phase 2 clinical trial reported that a therapy based on RNA interference (RNAi) showed some success in patients with liver fibrosis due to AAT deficiency.
Alpha1 antitrypsin deficiency disorder
Alpha1 antitrypsin deficiency is a rare genetic disorder that manifests mainly as lung disease and liver disease. AAT enzyme is an anti-protease enzyme that is encoded by the gene SERPINA1 and mainly synthesized in the liver.1,2 It reaches the lungs through blood circulation and functions as a protector against proteolytic enzymes.
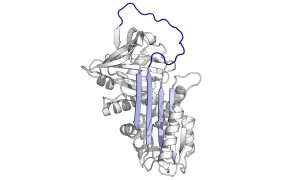
Caption: Alpha1-antitrypsin protein. Alpha1-antitrypsin deficiency is a rare genetic disorder that affects the lung and liver and is caused by mutations in a gene called SERPINA1. (Creative Commons image by Thomas Shafee, from https://en.wikipedia.org/wiki/Alpha-1_antitrypsin#/media/File:Alpha_1-antitrypsin.png)
More than 150 mutations in the SERPINA1 gene have been identified so far, with 95% of the severe cases of AAT deficiency, which are called Z-AAT deficiency, due to a single amino acid substitution, Glu342Lys. This mutation results in hepatocyte formation of a misfolded, trapped, and poorly secreted Z-AAT protein.
AAT deficiency symptoms
In the lungs, AAT deficiency causes the degeneration and destruction of the lung tissue, which frequently leads to the development of emphysema.

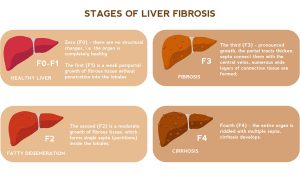
In the liver, AAT deficiency may cause jaundice, dark urine, enlarged liver, elevated liver enzymes, and poor growth in children.1 In adults, mutant AAT accumulation leads to hepatocellular injury, hepatitis, inflammation, fibrosis, and possibly cirrhosis (in 20 to 40% of cases).1,2 This can eventually lead to hepatocellular carcinoma.
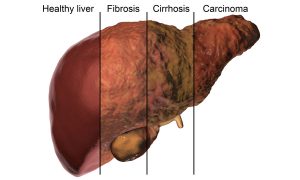
Caption: In the liver, AAT deficiency causes fibrosis and in 20 to 40% of cases causes cirrhosis, which can eventually lead to liver carcinoma. https://www.shutterstock.com/image-illustration/liver-disease-progression-hepatitis-b-c-690980266
AAT deficiency treatment
For AAT deficiency lung disease, treatment includes management of emphysema with standard treatments.1 Augmentation therapy is a treatment specific for AAT deficiency lung disease where patients are regularly infused with a purified, pooled AAT enzyme derived from human plasma.
There is no specific treatment for AAT deficiency liver disease, but research suggests that lowering the production of AAT enzyme or increasing the ability of the liver to break down AAT could reduce the risk of liver damage.1,2 Some patients require organ transplantation for end-stage liver disease or lung disease.
RNA interference therapeutics
One of the mechanisms that mammalian cells use to regulate gene expression is RNA interference (RNAi). The ability of RNAi to regulate gene expression resulted in the development of RNAi therapeutics as a relatively new class of drugs for the treatment of genetic, viral, metabolic, and degenerative diseases, as well as cancers.
Investigational RNAi therapy for AAT deficiency liver disease
In June 2022, Strnad and colleagues published in the New England Journal of Medicine the results of a multicenter, Phase 2, open-label clinical trial of an RNAi-based drug called fazirsiran for the treatment of liver fibrosis associated with SERPINA1 “Z” gene mutation.2 Fazirsiran is a synthetic, small interfering RNA (siRNA) duplex that binds messenger RNA (mRNA), causing its degradation and consequently the reduction of AAT synthesis by the liver.2,3
Patients in the trial were homozygous for the “Z” mutation and had a liver fibrosis staging between F1 and F3. Participants were divided into three cohorts: 1 (4 patients), 1b (4 patients), and 2 (8 patients).2 On day 1, week 4, and subsequently every 12 weeks, patients in cohorts 1 and 2 received 200 mg subcutaneous fazirsiran and those in cohort 1b received 100 mg subcutaneous fazirsiran.
The primary endpoint was the change from baseline in liver Z-AAT concentration in liver biopsy samples.2 Fazirsiran safety was also evaluated.
RNA interference study results
All evaluable patients (N = 14) had reductions in the accumulated total liver Z-AAT enzyme (83.3% median reduction, measured at week 24 in cohorts 1 and 1b and at week 48 in cohort 2).2 A significant mean reduction from baseline in serum Z-AAT concentration was also seen in all cohorts (a nadir reduction of about 90%) with the 200 mg cohort showing slightly greater sustained reductions up to week 52.
Fibrosis regression was observed in 7 out of 12 patients who received the 200 mg dose of fazirsiran but not in any of the patients who received the 100 mg dose. On the other hand, fibrosis progression was seen in two patients in cohort 2 by week 48.
Arthralgia and an increase in blood creatinine kinase were the most common adverse events during the study. Adverse events of viral myocarditis, diverticulitis, vestibular neuronitis, and dyspnea occurred in one patient each. No deaths or drug discontinuations occurred, and all serious adverse events were resolved.
Further studies will be required to follow up on this small, open-label trial. This clinical trial is one of several phase 2 and phase 3 trials involving RNAi therapeutics that are currently underway.3 In addition, three RNAi drugs have been approved by the FDA: patisiran for the treatment of genetic polyneuropathy, givosiran for the treatment of acute hepatic porphyria, and lumasiran for the treatment of primary hyperoxaluria type 1.
References:
- https://rarediseases.org/rare-diseases/alpha-1-antitrypsin-deficiency/
- https://www.nejm.org/doi/full/10.1056/NEJMoa2205416?query=TOC&cid=NEJM%20eToc,%20June%2030,%202022%20DM1205003_NEJM_Subscriber&bid=1047008304
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8946086/pdf/cancers-14-01588.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1978219/pdf/nihms-25447.pdf
- https://www.nature.com/articles/nchembio839.pdf

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.