The human immune system makes antibodies that bind to the same antigen with both of their variable regions, or “arms.” Monoclonal antibodies that replicate this basic design have been used in targeted cancer therapy since the late 1990s.1 Some of these antibodies bind to cancer cells and trigger an immune attack, while others enhance the activity of certain elements of the immune system. Another common strategy is to attach a radioactive agent or a chemotherapy drug to a monoclonal antibody. The antibody carries the toxic treatment directly to cancer cells, which allows it to attack the tumor with less risk of damage to non-targeted tissues.
Antibodies that bind to two different antigens are known as bispecific antibodies, while those that bind to three different antigens are called trispecific antibodies. Engineering and manufacturing these antibodies has traditionally been a challenge. However, recent advances in bispecific antibody development and manufacturing allow for new treatment strategies to be tested. Several bispecific monoclonal antibodies are in clinical trials or preclinical development for a variety of diseases, but they are of special interest in cancer treatment.
What is a bispecific monoclonal antibody?
Bispecific monoclonal antibodies, a part of the class of bifunctional molecules, are synthetic antibody-like proteins that can bind to two different antigens (targets) at once. Emicizumab and blinatumomab, the two bispecific antibodies are already on the market in the US. Emicizumab, a hemophilia A treatment with one arm that binds activated coagulation factor IX and one arm that binds factor X, leading to the activation of the latter. Emicizumab thus replicates the function of coagulation factor VIII, which is deficient in patients with hemophilia A. The drug is approved for routine prophylaxis of hemophilia A in the US and several other regions.
Bispecific antibodies for cancer therapy
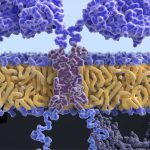
Bispecific antibodies for cancer fall under the umbrella of cancer immunotherapy. Currently, the only FDA-approved bispecific antibody for cancer therapy is blinatumomab, used in B-cell leukemia under certain circumstances. Blinatumomab is a synthetic antibody-like protein known as a bispecific T-cell engager (BiTE). BiTEs consist of two antibody-derived domains, one binds a surface protein found on tumor cells, and the other binds CD3, a surface protein on T cells. This simultaneously links the cells together and activates the T cell to attack the malignant cells.
Blinatumomab targets both CD3 and the B cell protein CD19, triggering T cells to attack malignant B cells. However, it also leads to depletion of normal B cells; cytokine release syndrome and neurologic toxicity can also occur. Blinatumomab has also been criticized for its high price, which was record-breaking for a cancer drug when it was first approved.
In addition to B-cell leukemia, blinatumomab is being investigated for use in other cancers, including indolent B-cell lymphoma and hairy cell leukemia, and as part of combination therapies.
Bispecific antibodies
Other bispecific antibodies are progressing through clinical trials. One of the most advanced products is tebotelimab, developed by Macrogenics. Tebotelimab is designed to bind to and inhibit two immune checkpoint molecules, PD-1 and LAG3, and thus restore the activity of T cells. It is currently in Phase II/III trial for gastric or gastroesophageal junction cancer and is also undergoing Phase I testing, alone or in combination with other drugs, for use in several other cancer types.
Glofitamab is under development by Roche for non-Hodgkin lymphomas. Glofitamab, the bispecific monoclonal antibody links T cells and B cells together, similarly to blinatumomab but using a different structure. A phase I trial has been completed, the findings showed significant response rates in patients with previously treated non-Hodgkin lymphomas.7 A phase III study in patients with diffuse large B-cell lymphoma and relapsed or refractory disease has been announced, and results are expected in 2022.
Meanwhile, bintrafusp alfa, a bifunctional fusion protein consisting of part of the TGF-β
(a TGF-β trap) protein fused to an antibody that blocks PD-L1 protein, produced disappointing results in a recent lung cancer trial. In March, it produced another disappointing outcome when it was investigated as second-line therapy for biliary tract cancer. However, bintrafusp alfa is still being tested as part of other trials, including a phase 2/3 trial of the agent as a first-line treatment in biliary tract cancer and another trial in human papillomavirus (HPV)-associated cancers, in which it is combined with two other agents.






1 Comment
CAR-T Therapy Approved for B-Cell Non-Hodgkin Lymphoma - MDforLives
5 years ago[…] the CAR-T strategy is similar to the one used by Amgen’s BiTE products, in which recombinant bispecific antibody-like proteins are used to physically link T cells to cancer cells and facilitate an attack. In the case of CAR-T, […]