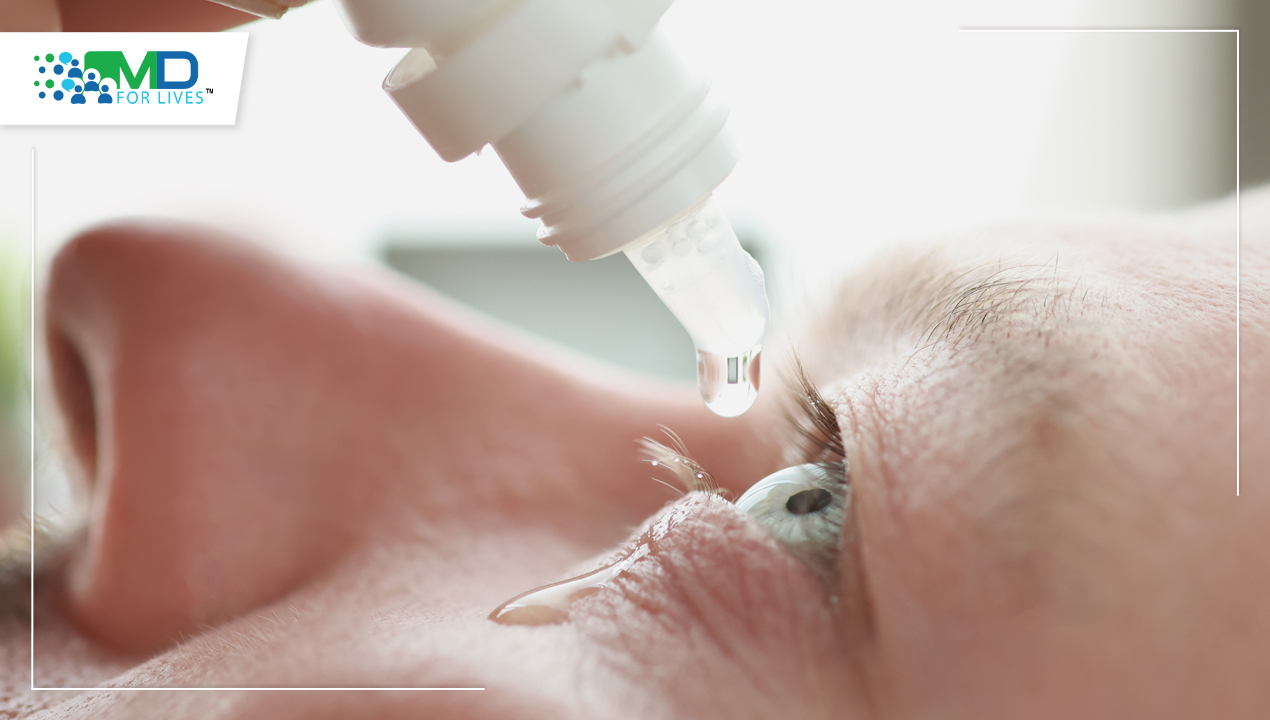
Dry eye treatment has become a central focus in ophthalmology as dry eye disease (DED) continues to rise globally. Once considered a mild, episodic condition, DED is now recognized as a chronic, multifactorial disease that significantly affects vision, comfort, and quality of life. Advances in diagnostics, a deeper understanding of disease mechanisms, and the emergence of targeted therapies have reshaped how clinicians approach management. This article reviews what dry eye disease is, why it is difficult to treat, and how the latest drugs, devices, and clinical studies are changing treatment strategies.
What Is Dry Eye Disease?
Dry eye disease is a disorder of the ocular surface characterized by loss of tear film homeostasis, accompanied by ocular symptoms such as discomfort, visual disturbance, and inflammation. Tear film instability, hyperosmolarity, inflammation, and neurosensory abnormalities all play a role in disease progression.
Types of Dry Eye Disease
Dry eye disease is broadly classified into two major types:
- Aqueous-deficient dry eye:
· Caused by reduced tear production from the lacrimal glands. This accounts for approximately 10–15% of cases.
- Evaporative dry eye:
Results from excessive tear evaporation, most commonly due to meibomian gland dysfunction (MGD). This is the dominant form of dry eye disease.
In clinical practice, many patients exhibit features of both types.
Symptoms and Causes
Common symptoms include:
- Dryness, burning, or stinging
- Foreign body sensation
- Redness
- Fluctuating or blurred vision
- Light sensitivity
Underlying causes include tear film instability, lipid layer deficiency, chronic inflammation, eyelid disorders, and environmental stressors.
Risk Factors for Dry Eyes
Risk factors include:
- Increasing age
- Female sex and hormonal changes
- Prolonged digital screen use
- Contact lens wear
- Autoimmune diseases
- Certain medications (antihistamines, antidepressants)
Complications of Dry Eyes
Untreated or severe dry eye disease can lead to:
- Chronic ocular surface inflammation
- Corneal epithelial damage
- Increased infection risk
- Reduced visual performance
Diagnosis
Diagnosis relies on a combination of symptoms and objective findings, including tear break-up time, ocular surface staining, Schirmer testing, and meibomian gland evaluation.
Dry Eye Treatment and Management
How Are Dry Eyes Treated?
Dry eye management follows a stepwise, severity-based approach, as recommended by TFOS DEWS II. Treatment is individualized and often requires combination therapy.
Dry Eye Syndrome Self-Care
Initial management includes:
- Patient education
- Environmental modification
- Lid hygiene and warm compresses
- Lubricating eye drops
These measures form the foundation of long-term disease control.
‘Deep Cleaning’ Devices for Dry Eye Treatment
Device-based therapies aim to restore meibomian gland function and improve tear film stability. Common approaches include:
- Thermal pulsation systems
- Manual meibomian gland expression
- Intense pulsed light (IPL)
These treatments are particularly useful for evaporative dry eye linked to MGD.
Why Is Dry Eye So Difficult to Treat?
Dry eye disease is challenging because it is:
- Chronic and relapsing
- Multifactorial rather than single cause
- Influenced by systemic, environmental, and behavioral factors
Symptoms often do not correlate perfectly with clinical signs, making treatment response variables.
Choosing the Best Lubricant Eye Drops for Dry Eyes
Artificial tears remain first-line therapy. Selection depends on:
- Preservative-free vs preserved formulations
- Viscosity and lipid content
- Frequency of use
No single formulation suits all patients, and trial-and-error is common.
How to Manage a Dry Eye Disease Flare-up
Flare-ups may require:
- Short-term topical corticosteroids
- Increased lubrication frequency
- Temporary activity modification
- Review of compliance and triggers
Early intervention can prevent progression to severe inflammation.
Remedies to Reduce Dry Eye Symptoms
Supportive measures include:
- Regular blinking during screen use
- Adequate hydration
- Omega-3 supplementation (where appropriate)
- Optimizing indoor humidity
Lifestyle modification plays a supportive, not standalone, role.
Latest Improved Dry Eye Drugs
Recent years have seen significant pharmacological advances:
- Cyclosporine formulations (0.05–0.1%)
Target immune-mediated inflammation and improve goblet cell density.
- Lifitegrast
Reduces T-cell–mediated inflammation at the ocular surface.
- Perfluorohexyloctane (NOV03 / Miebo)
Targets evaporative dry eye by stabilizing the lipid layer and reducing evaporation.
Dry Eye Disease Treatment – Latest Studies & Development
Recent clinical trials have expanded therapeutic options:
- NOV03 (Miebo) demonstrated early symptom relief and sustained improvement in evaporative dry eye.
- Lotilaner ophthalmic solution (TP-03) showed efficacy in treating Demodex-related blepharitis, a key contributor to dry eye.
- Reproxalap showed statistically significant improvement across symptom scores, tear osmolarity, and staining outcomes.
- AZR-MD-001 targeted meibomian gland obstruction with improvements in gland function and patient-reported symptoms.
These developments reflect a shift toward mechanism-specific treatment.
Advanced Dry Eye Therapies
Advanced options for refractory disease include:
- Punctal occlusion
- Autologous serum eye drops
- Bandage or scleral contact lenses
- Amniotic membrane therapy
- Surgical interventions in severe cases
Closing Perspective
Dry eye disease is no longer managed with lubrication alone. Advances in diagnostics, targeted pharmacotherapy, and device-based interventions have transformed treatment strategies. As understanding of tear film biology and ocular surface inflammation deepens, clinicians now have a broader and more precise toolkit to manage this complex condition. Ongoing research continues to refine therapies, offering improved outcomes for patients with both mild and severe disease.
Frequently Asked Questions
What Is the Most Effective Treatment for Dry Eyes?
There is no single best treatment. Management depends on disease subtype, severity, and underlying cause, often requiring combination therapy.
When to See a Doctor for Dry Eye Treatment?
Patients should seek evaluation if symptoms persist, worsen, or affect vision despite over-the-counter treatments.
What Are the Prescription Treatments for Dry Eye?
Prescription options include anti-inflammatory agents such as cyclosporine, lifitegrast, short-term corticosteroids, and newer lipid-targeting therapies.
What Are At-Home & Lifestyle Changes for Dry Eye Treatment?
Warm compresses, lid hygiene, environmental modification, and digital eye strain management support medical therapy.

MDForLives is a global healthcare intelligence platform where real-world perspectives are transformed into validated insights. We bring together diverse healthcare experiences to discover, share, and shape the future of healthcare through data-backed understanding.