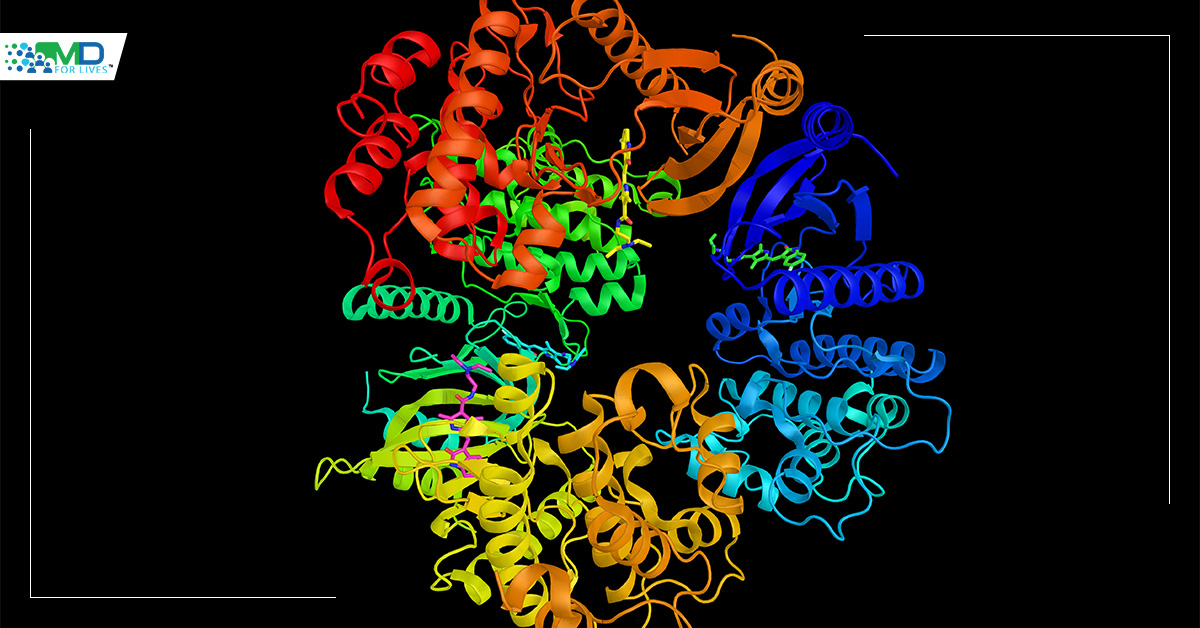
Neurotrophic Tyrosine Kinase genes (NTRK1, NTRK2 and NTRK3) encode members of the tyrosine receptor kinase (TRK) protein family: TRKA, TRKB, and TRKC, respectively. Genomic rearrangement in the NTRK genes leads to TRK fusion proteins that are involved in different pathways as you can see below (including PI3K, AKT, MTOR, RAS, RAF, MEK, ERK, PLCy, DAG, PKC). These pathways are critical for tumor cell proliferation, survival, invasion, and angiogenesis.

NTRK gene fusions act as oncogenic drivers of various tumors. The TRK kinase domain is always included in the oncogenic fusion protein, but the transmembrane domain is not always present and previous literature has found that it might have an effect on the cellular localization of the fusion protein (Cocco et al. 2018 PMID: 30333516. DOI: 10. 1038/s41571-018-0113-0). Below, you can see the activating mechanisms of NTRK fusions:
NTRK fusions can both be found in adult and child populations. They are usually observed at a very high frequency in specific rare tumors (depicted in the image below in red), with a frequency as high as 90% in specific patient cohorts. However, they are usually observed in low frequencies in most common cancer types, including lung cancer, breast cancer, and melanomas. The annual incidence of NTRK fusion-driven tumors is estimated to be 1500–5000 cases in the United States alone.
Below is the timeline of key advances relating to the biology and therapeutic targeting of TRK signaling, culminating in 2017’s FDA approval of the selective TRK inhibitors Larotrectinib (Nov 2018) and Entrectinib (August 2019) for both adult and child patient populations.

In a 2016–2019 study in the Children’s Hospital of Philadelphia, an in-house fusion panel (RNA based) was conducted, where 110 major fusion partners associated with over 600 known cancer fusions and many novel fusions were analyzed.

Utilizing an Archer™ Anchored Multiplex PCR (AMP™ technology – seen above), 1433 cases were tested from 1150 patients. NTRK fusions were detected in 25 tumors (1.6%) from 22 patients.
As you can see below, in total 10/25 fusions were novel or rarely reported in the literature for other tumor types. In 19 patients, the fusions identified confirmed the clinical/pathological diagnoses. In 3 patients, the diagnosis was changed due to the identification of an NTRK fusion.

NTRK fusions have been identified in 3% of solid tumors, and 1.6% of tumors amongst PTCs, soft-tissue sarcomas, brain tumors, and leukemia, further expanding the spectrum of NTRK-associated tumors.
In terms of treatments, larotrectnib and entrectinib will soon be standard of care for these patients, as they have acceptable data in both adult and pediatric patients.
For Larotrectinib, there have been 3 key trials: The LOXO-TRK-14001 trial (NCT02122913), assessing the safety of Larotrectinib in adult patients; SCOUT trial (NCT026376687), testing safety and efficacy in children; and the basket trial NAVIGATE (NCT02576431) evaluating safety and efficacy in both adult and adolescent patients. Patients were enrolled on the basis they had confirmed TRK fusion (identified by next-gen sequencing or a FISH panel), a noncentral nervous system primary tumor, and had received a dose of larotrectinib. The most common tumor types included salivary gland (22%), multiple soft-tissue sarcoma subtypes (20%), and infantile fibrosarcoma (13%), with an age range of 4 months to 76 years old. The overall response rate (evaluated by RECIST criteria) was 75%, with a median time to response of 1.8 months. Data of efficacy and adverse events can be seen below:

NCCN Guidelines 2020 update for NSCLC recommends larotrectinib and entrectinib as first-line and subsequent treatment options for patients with advanced or metastatic disease with NTRK gene fusion. Entrectinib has also been added as a therapeutic option for patients positive for ROS1 rearrangement.
In conclusion, it is clear through studies that NTRK fusions are oncogenic drivers and diagnostic markers for certain cancers including secretory breast carcinoma, congenital infantile fibrosarcoma, and mammary analogue secretory carcinoma. The FDA approval of TRK inhibitors for NTRK fusion positive tumors is an important milestone of tumor independent targeted therapies based on molecular profile. The identification of NTRK fusions is critical in pediatric cancer care as it provides genomic evidence for tumor diagnosis and targeted therapy for patients with relapsed or unresectable tumors.