No FDA-approved treatments yet exist for alopecia areata, a condition in which hair is lost in patches or from areas of the body. On May 5th, results from two phase 3 trials of a drug called baricitinib were published in the New England Journal of Medicine. The treatment showed significant benefits for alopecia patients, with more of the treated patients meeting a hair regrowth criterion compared to those on placebo. Baricitinib is a Janus kinase inhibitor – a member of a drug class used in several inflammatory, skin, and malignant conditions – and is currently FDA-approved for use in certain patients with rheumatoid arthritis.
What is Alopecia Areata?
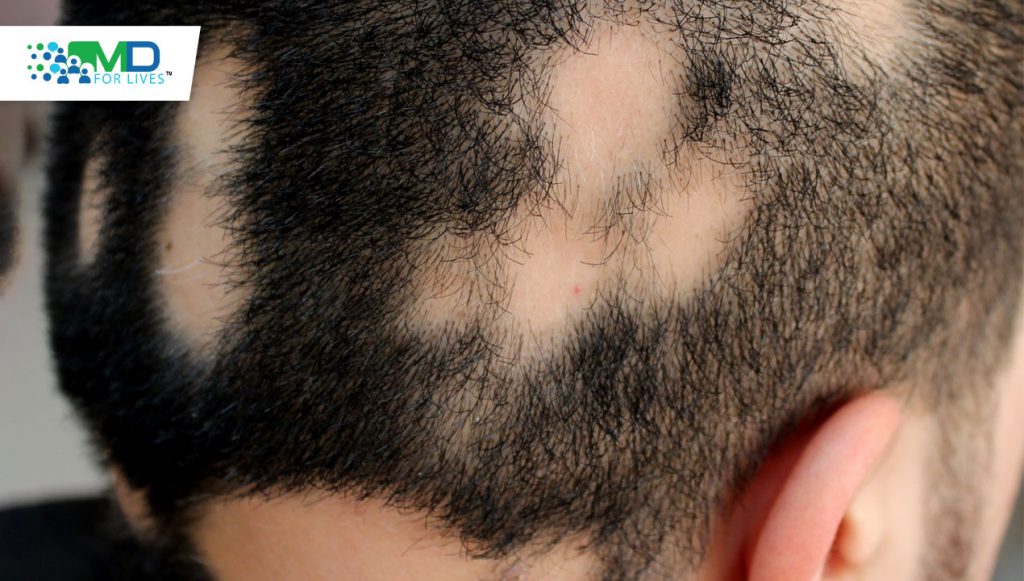
Alopecia areata involves an autoimmune attack on the hair follicles. The cause of this attack is not well understood but is an area of active research.
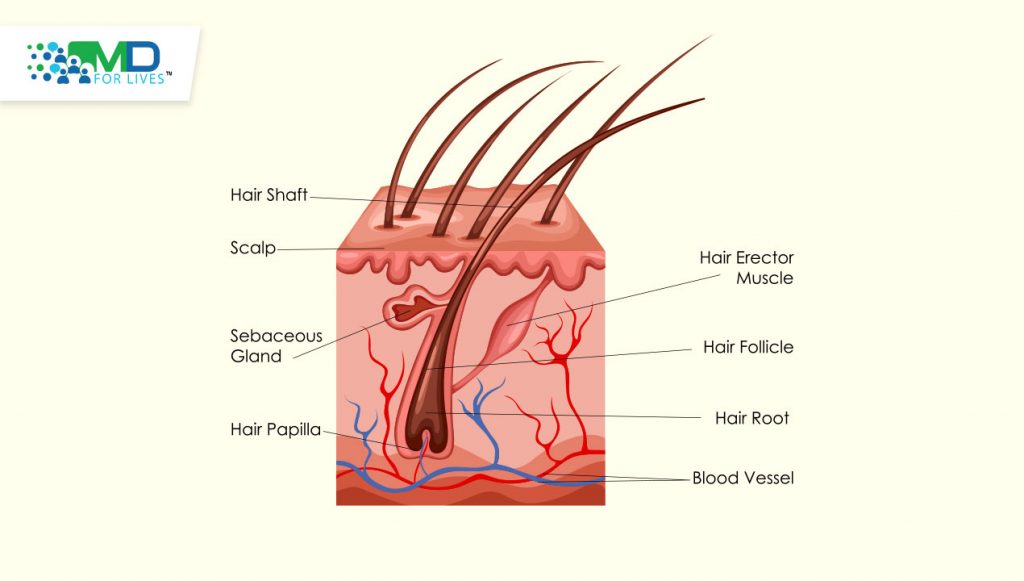
The scalp, eyelashes, and eyebrows are the areas most commonly affected by hair loss, but any area of the body can be affected. Some patients recover within a few months without treatment, but many others develop chronic alopecia. Hair can be lost in patches or from whole regions, and some patients develop alopecia universalis, in which all body hair is lost. Emotional and social consequences are common, and one study in the UK found that individuals with alopecia areata were more likely to develop depression and anxiety, and more likely to be unemployed.
Before the recent studies on baricitinib for alopecia, no alopecia areata treatment had been tested in a published Phase 3 trial. Commonly used treatments include corticosteroids, which can be used topically, intralesionally, or systemically, as well as topical immunotherapy agents, although none of these are FDA-approved for use in alopecia and they are not effective for all patients.
Janus Kinase Inhibitors for Alopecia
Janus kinases (JAKs) are tyrosine kinase proteins that are involved in cytokine signaling in the human body. Several drugs that target these proteins- Janus kinase inhibitors- have been approved by the FDA for treatment of rheumatoid arthritis, psoriasis, atopic dermatitis, polycythemia vera, and a few other conditions. What is the rationale for using Janus kinase inhibitors in alopecia? Research in 2014 suggested that two cytokines, interferon-γ and interleukin-15, are important in driving alopecia disease. Since Janus kinase proteins are involved in cytokine signaling, blocking them could potentially stop or even reverse the immune system’s attack on hair follicles.
Baricitinib inhibits both JAK1 and JAK2. Two other drugs, ritlecitinib and brepocitinib, inhibit different combinations of JAKs and other tyrosine kinase proteins, but all three have shown promise in treating alopecia areata in Phase 2 trials.
Two Trials of Baricitinib for Alopecia
The results of two double-blind, randomized, placebo-controlled trials of orally dosed baricitinib for alopecia were published on May 5, 2022. The trials were designed and sponsored by Eli Lilly, the manufacturer of baricitinib. The first trial, BRAVE-AA1, included 654 patients, and the second, BRAVE-AA2, included 546 patients; the participants lived in 10 countries.
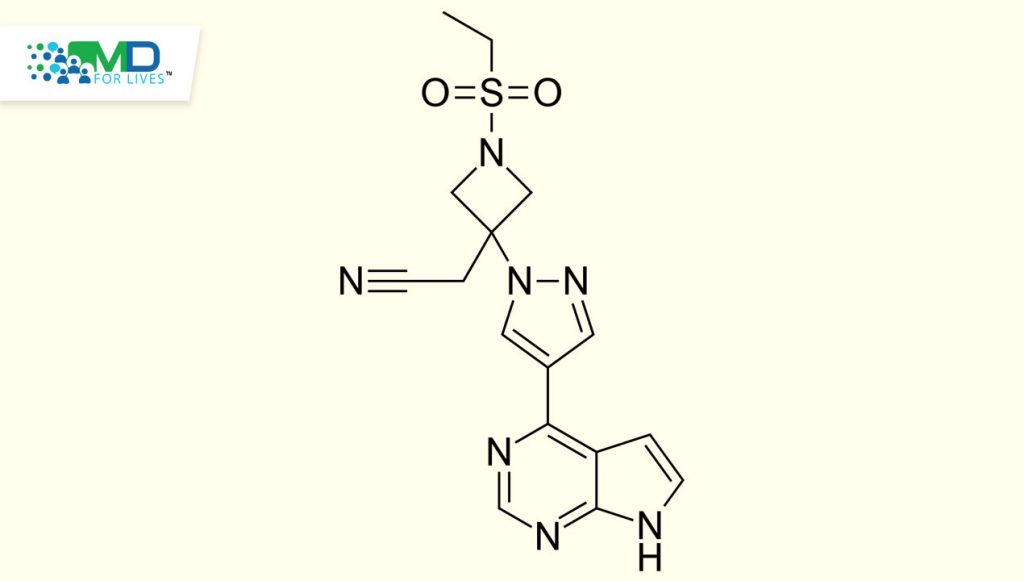
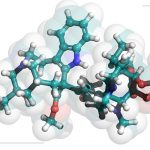
Caption: Structure of baricitinib, a Janus kinase inhibitor studied in two recent trials for alopecia areata.
All participants had alopecia areata that had persisted for at least 6 months, and had a current Severity of Alopecia Tool (SALT) score of 50 or more, indicating loss of at least 50% of scalp hair. Fifty-three percent of the participants had very severe alopecia, with loss of 95-100% of their scalp hair. The participants were randomly assigned in a 3:2:2 ratio to receive oral baricitinib at 4 mg daily, oral baricitinib at 2 mg daily, or placebo.
The investigators used multiple imputation because 10% to 15% of the data were missing for primary and secondary outcomes. Based on this analysis, both trials found significant differences between the placebo and each of the treatment doses in the percentage of patients achieving the primary outcome, which was a SALT score of 20 or less (indicating loss of 20% or less of scalp hair) after 36 weeks of treatment.
In BRAVE-AA2, of the patients on 4 mg of daily baricitinib, 38.8% had a SALT score of 20 or less, compared with 22.8% on 2 mg baricitinib and 6.2% on placebo. In BRAVE-AA2, 35.9% (4 mg baricitinib), 19.4% (2 mg baricitinib), and 3.35 (placebo) of patients attained a SALT score of 20 or less at week 36.
The trials also recorded potential baricitinib side effects. Adverse events that were more common with baricitinib included elevated creatine kinase levels, infections, acne, and increased LDL and HDL cholesterol levels.
One limitation of these studies is that they excluded patients who had previously failed to respond to an oral JAK inhibitor as well as patients whose alopecia had persisted more than 8 years without hair regrowth. As pointed out in an accompanying editorial in NEJM, JAK inhibitors are also expensive.
Additionally, these studies were relatively short-term, while many patients would likely have to take the medication long-term to prevent recurrent disease.1,2 In 2021, the FDA issued a warning regarding increased risks of heart-related adverse events with the use of certain JAK inhibitors in patients with inflammatory conditions.7 In the case of baricitinib for alopecia, further studies of long-term safety will be required; extension trials for BRAVE are currently ongoing.
Also read-
References
1. https://www.nejm.org/doi/full/10.1056/NEJMoa2110343?query=TOC
2. https://www.nejm.org/doi/full/10.1056/NEJMe2203440?query=TOC&cid=NEJM+eToc%2C+May+5%2C+2022+DM1008104_NEJM_Non_Subscriber&bid=960664880
3. https://academic.oup.com/rheumatology/article/58/Supplement_1/i43/5365419
4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8328385/
5. https://onlinelibrary.wiley.com/doi/10.1111/bjd.21055
6. https://www.medicalnewstoday.com/articles/70956#treatment
7. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death

MDForLives is a vibrant community of healthcare professionals and patients dedicated to shaping the future of healthcare. We provide valuable global insights to healthcare companies through online surveys, interviews, and discussion forums.